Mitochondrial Dysfunction and Alzheimer's Disease: Role of Microglia
- PMID: 32973488
- PMCID: PMC7468434
- DOI: 10.3389/fnagi.2020.00252
Mitochondrial Dysfunction and Alzheimer's Disease: Role of Microglia
Abstract
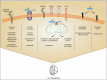
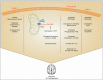
In 1907, Alois Alzheimer observed, as he quoted, development of "numerous fibers" and "adipose saccules" in the brain of his diseased patient Auguste Deter. The neurodegenerative disease became known as Alzheimer's disease (AD) and is the most common cause of dementia worldwide. AD normally develops with aging and is mostly initiated because of the imbalance between the formation and clearance of amyloid-β (Aβ). Formation of neurofibrillary tangles (NFTs) of hyperphosphorylated tau is another hallmark of AD. Neuroinflammation plays a significant role in the development and pathology of AD. This chapter explores the role of mitochondrial dysfunction in microglia in case of AD. Mitochondrial oxidative stress in microglia has been linked to the development of AD. Elevated generation of reactive oxygen species (ROS) and loss of mitochondrial membrane potential through various mechanisms have been observed in AD. Aβ interacts with microglial receptors, such as triggering receptor expressed in myeloid cells 2 (TREM2), activating downstream pathways causing mitochondrial damage and aggravating inflammation and cytotoxicity. Fibrillar Aβ activates nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in microglia leading to elevated induction of mitochondrial ROS which further causes neurotoxicity. Elevated ROS in microglia causes activation of inflammatory and cell death pathways. Production of ATP, regulation of mitochondrial health, autophagy, and mitophagy in microglia play significant roles in the AD pathology. Understanding microglial physiology and mitochondrial dysfunction will enable better therapeutic interventions.
Keywords: ROS; amyloid-β; microglia; mitochondria; neurodegeneration.
Copyright © 2020 Agrawal and Jha.
Figures
References
-
- Alzheimer’s Association (2018). 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 14, 367–429. 10.1016/j.jalz.2018.02.001 - DOI
Publication types
LinkOut - more resources
Full Text Sources

