The C-C Chemokine Receptor Type 4 Is an Immunomodulatory Target of Hydroxychloroquine
- PMID: 32973504
- PMCID: PMC7482581
- DOI: 10.3389/fphar.2020.01253
The C-C Chemokine Receptor Type 4 Is an Immunomodulatory Target of Hydroxychloroquine
Abstract
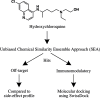
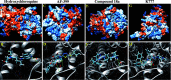
The emergence of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; COVID-19) in China, reported to the World Health Organization on December 31, 2019, has led to a large global pandemic and is a major public health issue. As a result, there are more than 200 clinical trials of COVID-19 treatments or vaccines that are either ongoing or recruiting patients. One potential therapy that has garnered international attention is hydroxychloroquine; a potent immunomodulatory agent FDA-approved for the treatment of numerous inflammatory and autoimmune conditions, including malaria, lupus, and rheumatoid arthritis. Hydroxychloroquine has demonstrated promise in vitro and is currently under investigation in clinical trials for the treatment of COVID-19. Despite an abundance of empirical data, the mechanism(s) involved in the immunomodulatory activity of hydroxychloroquine have not been characterized. Using the unbiased chemical similarity ensemble approach (SEA), we identified C-C chemokine receptor type 4 (CCR4) as an immunomodulatory target of hydroxychloroquine. The crystal structure of CCR4 was selected for molecular docking studies using the SwissDock modeling software. In silico, hydroxychloroquine interacts with Thr-189 within the CCR4 active site, presumably blocking endogenous ligand binding. However, the CCR4 antagonists compound 18a and K777 outperformed hydroxychloroquine in silico, demonstrating energetically favorable binding characteristics. Hydroxychloroquine may subject COVID-19 patients to QT-prolongation, increasing the risk of sudden cardiac death. The FDA-approved CCR4 antagonist mogalizumab is not known to increase the risk of QT prolongation and may serve as a viable alternative to hydroxychloroquine. Results from this report introduce additional FDA-approved drugs that warrant investigation for therapeutic use in the treatment of COVID-19.
Keywords: CCR4; COVID-19; SARS-CoV-2; hydroxychloroquine; immunomodulation.
Copyright © 2020 Beck, Beck, Holloway, Hemings, Dix and Norris.
Figures
Similar articles
-
A Trial of Favipiravir and Hydroxychloroquine combination in Adults Hospitalized with moderate and severe Covid-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 31;21(1):904. doi: 10.1186/s13063-020-04825-x. Trials. 2020. PMID: 33129363 Free PMC article.
-
Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19.Eur Rev Med Pharmacol Sci. 2020 Apr;24(8):4539-4547. doi: 10.26355/eurrev_202004_21038. Eur Rev Med Pharmacol Sci. 2020. PMID: 32373993 Review.
-
Toxicity of chloroquine and hydroxychloroquine following therapeutic use or overdose.Clin Toxicol (Phila). 2021 Jan;59(1):12-23. doi: 10.1080/15563650.2020.1817479. Epub 2020 Sep 22. Clin Toxicol (Phila). 2021. PMID: 32960100 Review.
-
Synergistic antiviral effect of hydroxychloroquine and azithromycin in combination against SARS-CoV-2: What molecular dynamics studies of virus-host interactions reveal.Int J Antimicrob Agents. 2020 Aug;56(2):106020. doi: 10.1016/j.ijantimicag.2020.106020. Epub 2020 May 13. Int J Antimicrob Agents. 2020. PMID: 32405156 Free PMC article.
-
Hydroxychloroquine in COVID-19 Therapy: Protection Versus Proarrhythmia.J Cardiovasc Pharmacol Ther. 2020 Nov;25(6):497-502. doi: 10.1177/1074248420935740. Epub 2020 Jul 23. J Cardiovasc Pharmacol Ther. 2020. PMID: 32700555 Review.
Cited by
-
Hydroxychloroquine attenuates autoimmune hepatitis by suppressing the interaction of GRK2 with PI3K in T lymphocytes.Front Pharmacol. 2022 Sep 15;13:972397. doi: 10.3389/fphar.2022.972397. eCollection 2022. Front Pharmacol. 2022. PMID: 36188529 Free PMC article.
-
The Laws of Attraction: Chemokines as Critical Mediators in Cancer Progression and Immunotherapy Response in Bladder Cancer.Cancers (Basel). 2024 Sep 27;16(19):3303. doi: 10.3390/cancers16193303. Cancers (Basel). 2024. PMID: 39409924 Free PMC article. Review.
-
A Narrative Review of the State of the Art of CCR4-Based Therapies in Cutaneous T-Cell Lymphomas: Focus on Mogamulizumab and Future Treatments.Antibodies (Basel). 2024 Apr 22;13(2):32. doi: 10.3390/antib13020032. Antibodies (Basel). 2024. PMID: 38804300 Free PMC article. Review.
-
Rapid, High-Throughput Single-Cell Multiplex In Situ Tagging (MIST) Analysis of Immunological Disease with Machine Learning.Anal Chem. 2023 May 16;95(19):7779-7787. doi: 10.1021/acs.analchem.3c01157. Epub 2023 May 4. Anal Chem. 2023. PMID: 37141575 Free PMC article.
-
In-silico discovery of type-2 diabetes-causing host key genes that are associated with the complexity of monkeypox and repurposing common drugs.Brief Bioinform. 2025 May 1;26(3):bbaf215. doi: 10.1093/bib/bbaf215. Brief Bioinform. 2025. PMID: 40370100 Free PMC article.
References
-
- Ankrum J. (2020). Can cell therapies halt cytokine storm in severe COVID-19 patients? Sci. Tranl. Med. 12 (540), eabb5673. 10.1126/scitranslmed.abb5673 - DOI
-
- Auerbach P. (2020). DrugMatrix in vitro pharmacology data (National Toxicology Program; ). Available at: https://ntp.niehs.nih.gov/drugmatrix/index.html (Accessed on July 22, 2020).
-
- Bayry J., Tchilian E. Z., Davies M. N., Forbes E. K., Draper S. J., Kaveri S. V., et al. (2008). In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc. Natl. Acad. Sci. U. S. A. 105(29), 10221–10226. 10.1073/pnas.0803453105 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

