Phagolysosomal Survival Enables Non-lytic Hyphal Escape and Ramification Through Lung Epithelium During Aspergillus fumigatus Infection
- PMID: 32973709
- PMCID: PMC7468521
- DOI: 10.3389/fmicb.2020.01955
Phagolysosomal Survival Enables Non-lytic Hyphal Escape and Ramification Through Lung Epithelium During Aspergillus fumigatus Infection
Abstract
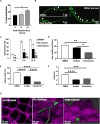
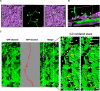
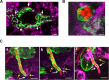
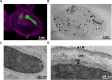
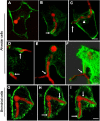
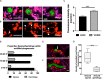
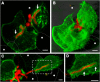
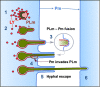
Aspergillus fumigatus is the most important mould pathogen in immunosuppressed patients. Suboptimal clearance of inhaled spores results in the colonisation of the lung airways by invasive hyphae. The first point of contact between A. fumigatus and the host is the lung epithelium. In vitro and ex vivo studies have characterised critical aspects of the interaction of invasive hyphae on the surface of epithelial cells. However, the cellular interplay between internalised A. fumigatus and the lung epithelium remains largely unexplored. Here, we use high-resolution live-cell confocal microscopy, 3D rendered imaging and transmission electron microscopy to define the development of A. fumigatus after lung epithelium internalisation in vitro. Germination, morphology and growth of A. fumigatus were significantly impaired upon internalisation by alveolar (A549) and bronchial (16HBE) lung epithelial cells compared to those growing on the host surface. Internalised spores and germlings were surrounded by the host phagolysosome membrane. Sixty per cent of the phagosomes containing germlings were not acidified at 24 h post infection allowing hyphal development. During escape, the phagolysosomal membrane was not ruptured but likely fused to host plasma membrane allowing hyphal exit from the intact host cell in an non-lytic Manner. Subsequently, escaping hyphae elongated between or through adjacent epithelial lung cells without penetration of the host cytoplasm. Hyphal tips penetrating new epithelial cells were surrounded by the recipient cell plasma membrane. Altogether, our results suggest cells of lung epithelium survive fungal penetration because the phagolysosomal and plasma membranes are never breached and that conversely, fungal spores survive due to phagosome maturation failure. Consequently, fungal hyphae can grow through the epithelial cell layer without directly damaging the host. These processes likely prevent the activation of downstream immune responses alongside limiting the access of professional phagocytes to the invading fungal hypha. Further research is needed to investigate if these events also occur during penetration of fungi in endothelial cells, fibroblasts and other cell types.
Keywords: Aspergillus fumigatus; airway epithelium; aspergillosis; epithelial cell infection; membrane compartmentalisation; microscopy; phagolysosome.
Copyright © 2020 Seidel, Moreno-Velásquez, Ben-Ghazzi, Gago, Read and Bowyer.
Figures
Similar articles
-
Use of a human small airway epithelial cell line to study the interactions of Aspergillus fumigatus with pulmonary epithelial cells.mSphere. 2023 Oct 24;8(5):e0031423. doi: 10.1128/msphere.00314-23. Epub 2023 Aug 14. mSphere. 2023. PMID: 37578262 Free PMC article.
-
Pulmonary defense mechanisms against opportunistic fungal pathogens.Immunol Ser. 1989;47:243-71. Immunol Ser. 1989. PMID: 2490078 Review.
-
Aspergillus fumigatus Cell Wall Promotes Apical Airway Epithelial Recruitment of Human Neutrophils.Infect Immun. 2020 Jan 22;88(2):e00813-19. doi: 10.1128/IAI.00813-19. Print 2020 Jan 22. Infect Immun. 2020. PMID: 31767773 Free PMC article.
-
Characterisation of Aspergillus fumigatus Endocytic Trafficking within Airway Epithelial Cells Using High-Resolution Automated Quantitative Confocal Microscopy.J Fungi (Basel). 2021 Jun 7;7(6):454. doi: 10.3390/jof7060454. J Fungi (Basel). 2021. PMID: 34200399 Free PMC article.
-
The innate immune response to Aspergillus fumigatus at the alveolar surface.FEMS Microbiol Rev. 2015 Sep;39(5):670-87. doi: 10.1093/femsre/fuv018. Epub 2015 Apr 30. FEMS Microbiol Rev. 2015. PMID: 25934117 Review.
Cited by
-
Stimulating the autophagic-lysosomal axis enhances host defense against fungal infection in a zebrafish model of invasive Aspergillosis.Autophagy. 2023 Jan;19(1):324-337. doi: 10.1080/15548627.2022.2090727. Epub 2022 Jul 1. Autophagy. 2023. PMID: 35775203 Free PMC article.
-
Manipulation of host phagocytosis by fungal pathogens and therapeutic opportunities.Nat Microbiol. 2024 Sep;9(9):2216-2231. doi: 10.1038/s41564-024-01780-0. Epub 2024 Aug 26. Nat Microbiol. 2024. PMID: 39187614 Review.
-
Novel Insights into Aspergillus fumigatus Pathogenesis and Host Response from State-of-the-Art Imaging of Host-Pathogen Interactions during Infection.J Fungi (Basel). 2022 Mar 4;8(3):264. doi: 10.3390/jof8030264. J Fungi (Basel). 2022. PMID: 35330266 Free PMC article. Review.
-
Fungal immunity and pathogenesis in mammals versus the invertebrate model organism Galleria mellonella.Pathog Dis. 2021 Mar 20;79(3):ftab013. doi: 10.1093/femspd/ftab013. Pathog Dis. 2021. PMID: 33544836 Free PMC article. Review.
-
Trans-cellular tunnels induced by the fungal pathogen Candida albicans facilitate invasion through successive epithelial cells without host damage.Nat Commun. 2022 Jun 30;13(1):3781. doi: 10.1038/s41467-022-31237-z. Nat Commun. 2022. PMID: 35773250 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

