Multifactorial Design of a Supramolecular Peptide Anti-IL-17 Vaccine Toward the Treatment of Psoriasis
- PMID: 32973764
- PMCID: PMC7461889
- DOI: 10.3389/fimmu.2020.01855
Multifactorial Design of a Supramolecular Peptide Anti-IL-17 Vaccine Toward the Treatment of Psoriasis
Abstract
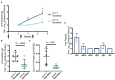
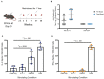
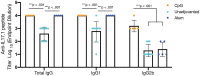
Current treatments for chronic immune-mediated diseases such as psoriasis, rheumatoid arthritis, or Crohn's disease commonly rely on cytokine neutralization using monoclonal antibodies; however, such approaches have drawbacks. Frequent repeated dosing can lead to the formation of anti-drug antibodies and patient compliance issues, and it is difficult to identify a single antibody that is broadly efficacious across diverse patient populations. As an alternative to monoclonal antibody therapy, anti-cytokine immunization is a potential means for long-term therapeutic control of chronic inflammatory diseases. Here we report a supramolecular peptide-based approach for raising antibodies against IL-17 and demonstrate its efficacy in a murine model of psoriasis. B-cell epitopes from IL-17 were co-assembled with the universal T-cell epitope PADRE using the Q11 self-assembling peptide nanofiber system. These materials, with or without adjuvants, raised antibody responses against IL-17. Exploiting the modularity of the system, multifactorial experimental designs were used to select formulations maximizing titer and avidity. In a mouse model of psoriasis induced by imiquimod, unadjuvanted nanofibers had therapeutic efficacy, which could be enhanced with alum adjuvant but reversed with CpG adjuvant. Measurements of antibody subclass induced by adjuvanted and unadjuvanted formulations revealed strong correlations between therapeutic efficacy and titers of IgG1 (improved efficacy) or IgG2b (worsened efficacy). These findings have important implications for the development of anti-cytokine active immunotherapies and suggest that immune phenotype is an important metric for eliciting therapeutic anti-cytokine antibody responses.
Keywords: active immunotherapy; anti-cytokine; immunoengineering; inflammatory diseases; self-assembly.
Copyright © 2020 Shores, Kelly, Hainline, Suwanpradid, MacLeod and Collier.
Figures




Similar articles
-
Anti-Cytokine Active Immunotherapy Based on Supramolecular Peptides for Alleviating IL-1β-Mediated Inflammation.Adv Healthc Mater. 2025 Feb;14(5):e2401444. doi: 10.1002/adhm.202401444. Epub 2024 Aug 7. Adv Healthc Mater. 2025. PMID: 39113323
-
Modular complement assemblies for mitigating inflammatory conditions.Proc Natl Acad Sci U S A. 2021 Apr 13;118(15):e2018627118. doi: 10.1073/pnas.2018627118. Proc Natl Acad Sci U S A. 2021. PMID: 33876753 Free PMC article.
-
Active immunotherapy for C5a-mediated inflammation using adjuvant-free self-assembled peptide nanofibers.Acta Biomater. 2024 Apr 15;179:83-94. doi: 10.1016/j.actbio.2024.02.042. Epub 2024 Mar 5. Acta Biomater. 2024. PMID: 38447809 Free PMC article.
-
IL-17 for therapy.J Dermatol Sci. 2017 Sep;87(3):221-227. doi: 10.1016/j.jdermsci.2017.06.010. Epub 2017 Jun 15. J Dermatol Sci. 2017. PMID: 28633806 Review.
-
IL-17 and IL-17R: an auspicious therapeutic target for psoriatic disease.Actas Dermosifiliogr. 2014 Oct;105 Suppl 1:21-33. doi: 10.1016/S0001-7310(14)70015-8. Actas Dermosifiliogr. 2014. PMID: 25398489 Review.
Cited by
-
Peptide-based supramolecular vaccine systems.Acta Biomater. 2021 Oct 1;133:153-167. doi: 10.1016/j.actbio.2021.05.003. Epub 2021 May 16. Acta Biomater. 2021. PMID: 34010691 Free PMC article. Review.
-
Self-assembling peptide nanofiber HIV vaccine elicits robust vaccine-induced antibody functions and modulates Fc glycosylation.Sci Adv. 2022 Sep 23;8(38):eabq0273. doi: 10.1126/sciadv.abq0273. Epub 2022 Sep 23. Sci Adv. 2022. PMID: 36149967 Free PMC article.
-
From structure to application: Progress and opportunities in peptide materials development.Curr Opin Chem Biol. 2021 Oct;64:131-144. doi: 10.1016/j.cbpa.2021.06.006. Epub 2021 Jul 29. Curr Opin Chem Biol. 2021. PMID: 34329941 Free PMC article. Review.
-
Transdermal Delivery of Therapeutic Compounds With Nanotechnological Approaches in Psoriasis.Front Bioeng Biotechnol. 2022 Jan 24;9:804415. doi: 10.3389/fbioe.2021.804415. eCollection 2021. Front Bioeng Biotechnol. 2022. PMID: 35141215 Free PMC article. Review.
-
Biomaterial engineering strategies for B cell immunity modulations.Biomater Sci. 2024 Apr 16;12(8):1981-2006. doi: 10.1039/d3bm01841e. Biomater Sci. 2024. PMID: 38456305 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

