Differential Roles of IDO1 and IDO2 in T and B Cell Inflammatory Immune Responses
- PMID: 32973768
- PMCID: PMC7461966
- DOI: 10.3389/fimmu.2020.01861
Differential Roles of IDO1 and IDO2 in T and B Cell Inflammatory Immune Responses
Abstract
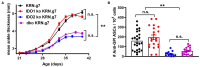
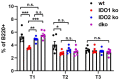
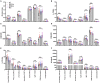
Indoleamine-2,3-dioxygenase (IDO)1 and IDO2 are two closely related tryptophan catabolizing enzymes encoded by linked genes. The IDO pathway is also immunomodulatory, with IDO1 well-characterized as a mediator of tumor immune evasion. Due to its homology with IDO1, IDO2 has been proposed to have a similar immunoregulatory function. Indeed, IDO2, like IDO1, is necessary for the differentiation of regulatory T cells in vitro. However, compared to IDO1, in vivo studies demonstrated a contrasting role for IDO2, with experiments in preclinical models of autoimmune arthritis establishing a proinflammatory role for IDO2 in mediating B and T cell activation driving autoimmune disease. Given their potentially opposing roles in inflammatory responses, interpretation of results obtained using IDO1 or IDO2 single knockout mice could be complicated by the expression of the other enzyme. Here we use IDO1 and IDO2 single and double knockout (dko) mice to define the differential roles of IDO1 and IDO2 in B cell-mediated immune responses. Autoreactive T and B cell responses and severity of joint inflammation were decreased in IDO2 ko, but not IDO1 ko arthritic mice. Dko mice had a reduction in the number of autoantibody secreting cells and severity of arthritis: however, percentages of differentiated T cells and their associated cytokines were not reduced compared to IDO1 ko or wild-type mice. These data suggest that autoreactive B cell responses are mediated by IDO2, while autoreactive T cell responses are indirectly affected by IDO1 expression in the IDO2 ko mice. IDO2 also influenced antibody responses in models of influenza infection and immunization with T cell-independent type II antigens. Taken together, these studies provide evidence for the contrasting roles IDO1 and IDO2 play in immune responses, with IDO1 mediating T cell suppressive effects and IDO2 working directly in B cells as a proinflammatory mediator of B cell responses.
Keywords: B cells; experimental arthritis; immunization; influenza; murine model.
Copyright © 2020 Merlo, DuHadaway, Montgomery, Peng, Murray, Prendergast, Caton, Muller and Mandik-Nayak.
Figures


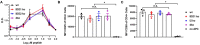


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

