Critical Neurotransmitters in the Neuroimmune Network
- PMID: 32973771
- PMCID: PMC7472989
- DOI: 10.3389/fimmu.2020.01869
Critical Neurotransmitters in the Neuroimmune Network
Abstract
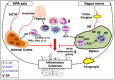
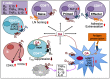
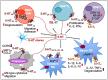
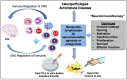
Immune cells rely on cell-cell communication to specify and fine-tune their responses. They express an extensive network of cell communication modes, including a vast repertoire of cell surface and transmembrane receptors and ligands, membrane vesicles, junctions, ligand and voltage-gated ion channels, and transporters. During a crosstalk between the nervous system and the immune system these modes of cellular communication and the downstream signal transduction events are influenced by neurotransmitters present in the local tissue environments in an autocrine or paracrine fashion. Neurotransmitters thus influence innate and adaptive immune responses. In addition, immune cells send signals to the brain through cytokines, and are present in the brain to influence neural responses. Altered communication between the nervous and immune systems is emerging as a common feature in neurodegenerative and immunopathological diseases. Here, we present the mechanistic frameworks of immunostimulatory and immunosuppressive effects critical neurotransmitters - dopamine (3,4-dihydroxyphenethylamine), serotonin (5-hydroxytryptamine), substance P (trifluoroacetate salt powder), and L-glutamate - exert on lymphocytes and non-lymphoid immune cells. Furthermore, we discuss the possible roles neurotransmitter-driven neuroimmune networks play in the pathogenesis of neurodegenerative disorders, autoimmune diseases, cancer, and outline potential clinical implications of balancing neuroimmune crosstalk by therapeutic modulation.
Keywords: T cell neuroimmunology; cancer; dopamine; glutamate; immunotherapy; neurodegenerative disorders; serotonin; substance P.
Copyright © 2020 Hodo, de Aquino, Shimamoto and Shanker.
Figures

References
-
- Jerne NK. Towards a network theory of the immune system. Ann Immunol. (1974) 125C:373–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

