Bimekizumab, a Novel Humanized IgG1 Antibody That Neutralizes Both IL-17A and IL-17F
- PMID: 32973785
- PMCID: PMC7473305
- DOI: 10.3389/fimmu.2020.01894
Bimekizumab, a Novel Humanized IgG1 Antibody That Neutralizes Both IL-17A and IL-17F
Abstract
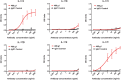
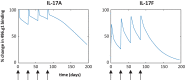
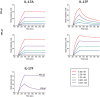
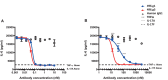
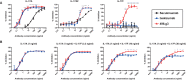
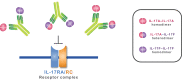
Interleukin (IL)-17A is a key driver of inflammation and the principal target of anti-IL-17 therapeutic monoclonal antibodies. IL-17A, and its structurally similar family member IL-17F, have been shown to be functionally dysregulated in certain human immune-mediated inflammatory diseases such as psoriasis, psoriatic arthritis, and axial spondyloarthritis. Given the overlapping biology of these two cytokines, we postulated that dual neutralization of IL-17A and IL-17F may provide a greater depth of clinical response in IL-17-mediated diseases than IL-17A inhibition alone. We identified 496.g1, a humanized antibody with strong affinity for IL-17A but poor affinity for IL-17F. Affinity maturation of 496.g1 to 496.g3 greatly enhanced the affinity of the Fab fragment for IL-17F while retaining strong binding to IL-17A. As an IgG1, the affinity for IL-17A and IL-17F was 3.2 pM and 23 pM, respectively. Comparison of 496.g3 IgG1 with the commercially available anti-IL-17A monoclonal antibodies ixekizumab and secukinumab, by surface plasmon resonance and in a human in vitro IL-17A functional assay, showed that 496.g3 and ixekizumab display equivalent affinity for IL-17A, and that both antibodies are markedly more potent than secukinumab. In contrast to ixekizumab and secukinumab, 496.g3 exhibited the unique feature of also being able to neutralize the biological activity of IL-17F. Therefore, antibody 496.g3 was selected for clinical development for its ability to neutralize the biologic function of both IL-17A and IL-17F and was renamed bimekizumab (formerly UCB4940). Early clinical data in patients with psoriasis, in those with psoriatic arthritis, and from the Phase 2 studies in psoriasis, psoriatic arthritis, and ankylosing spondylitis, are encouraging and support the targeted approach of dual neutralization of IL-17A and IL-17F. Taken together, these findings provide the rationale for the continued clinical evaluation of bimekizumab in patients with immune-mediated inflammatory diseases.
Keywords: IL-17A; IL-17F; anti-IL-17A; bimekizumab; dual neutralization; dual targeting; monoclonal antibody.
Copyright © 2020 Adams, Maroof, Baker, Lawson, Oliver, Paveley, Rapecki, Shaw, Vajjah, West and Griffiths.
Figures
References
-
- Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. (1993) 150:5445–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

