Effector CD8 T Cell-Dependent Zika Virus Control in the CNS: A Matter of Time and Numbers
- PMID: 32973802
- PMCID: PMC7461798
- DOI: 10.3389/fimmu.2020.01977
Effector CD8 T Cell-Dependent Zika Virus Control in the CNS: A Matter of Time and Numbers
Abstract
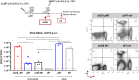
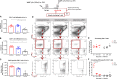
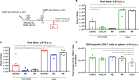
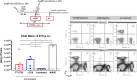
Zika virus (ZIKV), a mosquito-borne flavivirus, came into the spotlight in 2016 when it was found to be associated with an increased rate of microcephalic newborns in Brazil. The virus has further been recognized to cause neurologic complications in children and adults in the form of myelitis, encephalitis, acute disseminated encephalomyelitis (ADEM) and Guillain Barre Syndrome in a fraction of infected individuals. With the ultimate goal of identifying correlates of protection to guide the design of an effective vaccine, the study of the immune response to ZIKV infection has become the focus of research worldwide. Both innate and adaptive immune responses seem to be essential for controlling the infection. Induction of sufficient levels of neutralizing antibodies has been strongly correlated with protection against reinfection in various models, while the role of CD8 T cells as antiviral effectors in the CNS has been controversial. In an attempt to improve our understanding regarding the role of ZIKV-induced CD8 T cells in protective immunity inside the CNS, we have expanded on previous studies in intracranially infected mice. In a recent study, we have demonstrated that, peripheral ZIKV infection in adult C57BL/6 mice induces a robust CD8 T cell response that peaks within a week. In the present study, we used B cell deficient as well as wild-type mice to show that there is a race between CXCR3-dependent recruitment of the effector CD8 T cells and local ZIKV replication, and that CD8 T cells are capable of local viral control if they arrive in the brain early after viral invasion, in appropriate numbers and differentiation state. Our data highlight the benefits of considering this subset when designing vaccines against Zika virus.
Keywords: B cells; T cells; Zika virus; adaptive immunity; memory; mouse model; protection.
Copyright © 2020 Nazerai, Schøller, Bassi, Buus, Stryhn, Christensen and Thomsen.
Figures






References
-
- Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg. (1964) 58:335–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

