Pioneering Study on Rhopalurus crassicauda Scorpion Venom: Isolation and Characterization of the Major Toxin and Hyaluronidase
- PMID: 32973807
- PMCID: PMC7468477
- DOI: 10.3389/fimmu.2020.02011
Pioneering Study on Rhopalurus crassicauda Scorpion Venom: Isolation and Characterization of the Major Toxin and Hyaluronidase
Abstract
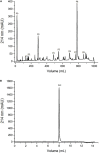
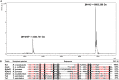
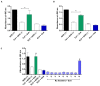
Scorpionism is responsible for most accidents involving venomous animals in Brazil, which leads to severe symptoms that can evolve to death. Scorpion venoms consist of complexes cocktails, including peptides, proteins, and non-protein compounds, making separation and purification procedures extremely difficult and time-consuming. Scorpion toxins target different biological systems and can be used in basic science, for clinical, and biotechnological applications. This study is the first to explore the venom content of the unexplored scorpion species Rhopalurus crassicauda, which inhabits exclusively the northernmost state of Brazil, named Roraima, and southern region of Guyana. Here, we pioneer the fractionation of the R. crassicauda venom and isolated and characterized a novel scorpion beta-neurotoxin, designated Rc1, and a monomeric hyaluronidase. R. crassicauda venom and Rc1 (6,882 Da) demonstrated pro-inflammatory activities in vitro and a nociceptive response in vivo. Moreover, Rc1 toxin showed specificity for activating Nav1.4, Nav1.6, and BgNav1 voltage-gated ion channels. This study also represents a new perspective for the treatment of envenomings in Roraima, since the Brazilian scorpion and arachnid antivenoms were not able to recognize R. crassicauda venom and its fractions (with exception of hyaluronidase). Our work provides useful insights for the first understanding of the painful sting and pro-inflammatory effects associated with R. crassicauda envenomings.
Keywords: Rhopalurus crassicauda; electrophysiology; neurotoxin; nociception; pro-inflammatory toxin; scorpion venom; toxin.
Copyright © 2020 Abreu, Bordon, Cerni, Oliveira, Balenzuela, Alexandre-Silva, Zoccal, Reis, Wiezel, Peigneur, Pinheiro-Júnior, Tytgat, Cunha, Quinton, Faccioli, Arantes, Zottich and Pucca.
Figures


Similar articles
-
Assessment of immunogenic characteristics of Hemiscorpius lepturus venom and its cross-reactivity with venoms from Androctonus crassicauda and Mesobuthus eupeus.J Immunotoxicol. 2015 Jul-Sep;12(3):217-22. doi: 10.3109/1547691X.2014.927542. Epub 2014 Jun 20. J Immunotoxicol. 2015. PMID: 24946724
-
Androctonus crassicauda (Olivier), a dangerous and unduly neglected scorpion--I. Pharmacological and clinical studies.Toxicon. 1994 Dec;32(12):1599-618. doi: 10.1016/0041-0101(94)90319-0. Toxicon. 1994. PMID: 7725329
-
Expression and purification of recombinant alpha-toxin AnCra1 from the scorpion Androctonus crassicauda and its functional characterization on mammalian sodium channels.Mol Biol Rep. 2021 Sep;48(9):6303-6312. doi: 10.1007/s11033-021-06624-2. Epub 2021 Aug 11. Mol Biol Rep. 2021. PMID: 34379289
-
Scorpions and scorpion sting envenoming (scorpionism) in the Arab Countries of the Middle East.Toxicon. 2021 Feb;191:83-103. doi: 10.1016/j.toxicon.2020.12.017. Epub 2020 Dec 31. Toxicon. 2021. PMID: 33387549 Review.
-
Biology, venom composition, and scorpionism induced by brazilian scorpion Tityus stigmurus (Thorell, 1876) (Scorpiones: Buthidae): A mini-review.Toxicon. 2020 Oct 15;185:36-45. doi: 10.1016/j.toxicon.2020.06.015. Epub 2020 Jul 6. Toxicon. 2020. PMID: 32585220 Review.
Cited by
-
Prognostic role of nutritional status in elderly patients hospitalized for COVID-19: a monocentric study.Aging Clin Exp Res. 2020 Dec;32(12):2695-2701. doi: 10.1007/s40520-020-01727-5. Epub 2020 Oct 8. Aging Clin Exp Res. 2020. PMID: 33034016 Free PMC article.
-
Scorpion Venom as a Source of Antimicrobial Peptides: Overview of Biomolecule Separation, Analysis and Characterization Methods.Antibiotics (Basel). 2023 Aug 29;12(9):1380. doi: 10.3390/antibiotics12091380. Antibiotics (Basel). 2023. PMID: 37760677 Free PMC article. Review.
-
Scorpion envenomation in Brazil: Current scenario and perspectives for containing an increasing health problem.PLoS Negl Trop Dis. 2023 Feb 9;17(2):e0011069. doi: 10.1371/journal.pntd.0011069. eCollection 2023 Feb. PLoS Negl Trop Dis. 2023. PMID: 36757916 Free PMC article. Review.
-
Antifungal activity of Rhopalurus crassicauda venom against Candida spp.Toxicon X. 2022 Mar 23;14:100120. doi: 10.1016/j.toxcx.2022.100120. eCollection 2022 Jun. Toxicon X. 2022. PMID: 35345480 Free PMC article.
-
Scorpion species of medical importance in the Brazilian Amazon: a review to identify knowledge gaps.J Venom Anim Toxins Incl Trop Dis. 2021 Sep 20;27:e20210012. doi: 10.1590/1678-9199-JVATITD-2021-0012. eCollection 2021. J Venom Anim Toxins Incl Trop Dis. 2021. PMID: 34589120 Free PMC article.
References
-
- Chippaux JP, Goyffon M. Venomous and poisonous animals – I. Overview. Med Trop. (2006) 66:215–20. - PubMed
-
- Souza WMP, Alexandre-Silva G, Cerni FA, Oliveira IS, Zottich U, Bassoli BK, et al. Envenomings caused by venomous animals in Roraima: a neglected health problem in the Brazil’s Northernmost state. TCR. (2019) 3:1–8. 10.24966/TCR-3735/100011 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

