Atypical Retinal Phenotype in a Patient With Alström Syndrome and Biallelic Novel Pathogenic Variants in ALMS1, Including a de novo Variation
- PMID: 32973878
- PMCID: PMC7472914
- DOI: 10.3389/fgene.2020.00938
Atypical Retinal Phenotype in a Patient With Alström Syndrome and Biallelic Novel Pathogenic Variants in ALMS1, Including a de novo Variation
Abstract
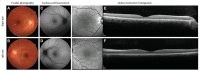
Alström syndrome (ALMS) is a rare autosomal recessive multi-organ syndrome considered to date as a ciliopathy and caused by variations in ALMS1. Phenotypic variability is well-documented, particularly for the systemic disease manifestations; however, early-onset progressive retinal degeneration affecting both cones and rods (cone-rod type) is universal, leading to blindness by the teenage years. Other features include cardiomyopathy, kidney dysfunction, sensorineural deafness, and childhood obesity associated with hyperinsulinemia and type 2 diabetes mellitus. Here, we present an unusual and delayed retinal dystrophy phenotype associated with ALMS in a 14-year-old female, with affected cone function and surprising complete preservation of rod function on serial electroretinograms (ERGs). High-throughput sequencing of the affected proband revealed compound heterozygosity with two novel nonsense variations in the ALMS1 gene, including one variant of de novo inheritance, an unusual finding in autosomal recessive diseases. To confirm the diagnosis in the context of an unusually mild phenotype and identification of novel variations, we demonstrated the biallelic status of the compound heterozygous variations (c.[286C > T];[1211C > G], p.[(Gln96*)];[(Ser404*)]). This unique case extends our knowledge of the phenotypic variability and the pathogenic variation spectrum in ALMS patients.
Keywords: ALMS1 gene; Alström syndrome; de novo variation; high throughput sequencing; retinal dystrophy.
Copyright © 2020 Mauring, Porter, Pelletier, Riehm, Leuvrey, Gouronc, Studer, Stoetzel, Dollfus and Muller.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

