Assessment of Visual and Retinal Function Following In Vivo Genipin-Induced Scleral Crosslinking
- PMID: 32974080
- PMCID: PMC7488211
- DOI: 10.1167/tvst.9.10.8
Assessment of Visual and Retinal Function Following In Vivo Genipin-Induced Scleral Crosslinking
Abstract
Purpose: Genipin has been proposed as a possible neuroprotective therapy in myopia and glaucoma. Here, we aim to determine the effects of prolonged genipin-induced scleral stiffening on visual function.
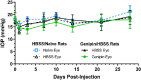
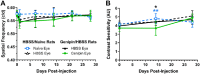
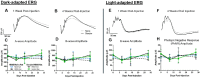
Methods: Eyes from Brown Norway rats were treated in vivo with either a single 15 mM genipin retrobulbar injection or sham retrobulbar injection and were compared to naïve eyes. Intraocular pressure, optomotor response, and electroretinograms were repeatedly measured over 4 weeks following retrobulbar injections to determine visual and retinal function. At 4 weeks, we quantified retinal ganglion cell axon counts. Finally, molecular changes in gene and protein expression were analyzed via real-time polymerase chain reaction (RT-PCR) and proteomics.
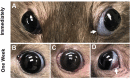
Results: Retrobulbar injection of genipin did not affect intraocular pressure (IOP) or retinal function, nor have a sustained impact on visual function. Although genipin-treated eyes had a small decrease in retinal ganglion cell axon counts compared to contralateral sham-treated eyes (-8,558 ± 18,646; mean ± SD), this was not statistically significant (P = 0.206, n = 9). Last, we did not observe any changes in gene or protein expression due to genipin treatment.
Conclusions: Posterior scleral stiffening with a single retrobulbar injection of 15 mM genipin causes no sustained deficits in visual or retinal function or at the molecular level in the retina and sclera. Retinal ganglion cell axon morphology appeared normal.
Translational significance: These results support future in vivo studies to determine the efficacy of genipin-induced posterior scleral stiffening to help treat ocular diseases, like myopia and glaucoma.
Keywords: crosslinking; genipin; glaucoma; myopia; scleral stiffening.
Copyright 2020 The Authors.
Conflict of interest statement
Disclosure: B.G. Hannon, None; C. Luna, None; A.J. Feola, None; M.D. Ritch, None; A.T. Read, None; S.S. Stinnett, None; H. Vo, None; M.T. Pardue, None; P. Ethier, None
Figures


Similar articles
-
Sustained scleral stiffening in rats after a single genipin treatment.J R Soc Interface. 2019 Oct 31;16(159):20190427. doi: 10.1098/rsif.2019.0427. Epub 2019 Oct 16. J R Soc Interface. 2019. PMID: 31615330 Free PMC article.
-
Scleral crosslinking using genipin can compromise retinal structure and function in tree shrews.Exp Eye Res. 2022 Jun;219:109039. doi: 10.1016/j.exer.2022.109039. Epub 2022 Mar 24. Exp Eye Res. 2022. PMID: 35339475 Free PMC article.
-
Evaluation of Spatially Targeted Scleral Stiffening on Neuroprotection in a Rat Model of Glaucoma.Transl Vis Sci Technol. 2022 May 2;11(5):7. doi: 10.1167/tvst.11.5.7. Transl Vis Sci Technol. 2022. PMID: 35536721 Free PMC article.
-
[Aiming for zero blindness].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):168-93; discussion 194. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854109 Review. Japanese.
-
[A challenge to primary open-angle glaucoma including normal-pressure. Clinical problems and their scientific solution].Nippon Ganka Gakkai Zasshi. 2012 Mar;116(3):233-67; discussion 268. Nippon Ganka Gakkai Zasshi. 2012. PMID: 22568103 Review. Japanese.
Cited by
-
Scleral Cross-Linking in Form-Deprivation Myopic Guinea Pig Eyes Leads to Glaucomatous Changes.Invest Ophthalmol Vis Sci. 2022 May 2;63(5):24. doi: 10.1167/iovs.63.5.24. Invest Ophthalmol Vis Sci. 2022. PMID: 35594036 Free PMC article.
-
Effects of Genipin Crosslinking of Porcine Perilimbal Sclera on Mechanical Properties and Intraocular Pressure.Bioengineering (Basel). 2024 Oct 2;11(10):996. doi: 10.3390/bioengineering11100996. Bioengineering (Basel). 2024. PMID: 39451372 Free PMC article.
-
Using retinal function to define ischemic exclusion criteria for animal models of glaucoma.Exp Eye Res. 2021 Jan;202:108354. doi: 10.1016/j.exer.2020.108354. Epub 2020 Nov 7. Exp Eye Res. 2021. PMID: 33171192 Free PMC article.
-
Effect of Scleral Crosslinking Using Multiple Doses of Genipin on Experimental Progressive Myopia in Tree Shrews.Transl Vis Sci Technol. 2021 Apr 29;10(5):1. doi: 10.1167/tvst.10.5.1. Transl Vis Sci Technol. 2021. PMID: 34003978 Free PMC article.
-
AxoNet 2.0: A Deep Learning-Based Tool for Morphometric Analysis of Retinal Ganglion Cell Axons.Transl Vis Sci Technol. 2023 Mar 1;12(3):9. doi: 10.1167/tvst.12.3.9. Transl Vis Sci Technol. 2023. PMID: 36917117 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

