From Sperm Motility to Sperm-Borne microRNA Signatures: New Approaches to Predict Male Fertility Potential
- PMID: 32974342
- PMCID: PMC7471662
- DOI: 10.3389/fcell.2020.00791
From Sperm Motility to Sperm-Borne microRNA Signatures: New Approaches to Predict Male Fertility Potential
Abstract
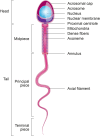
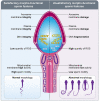
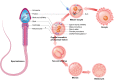
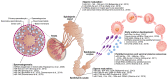
In addition to the paternal genome, spermatozoa carry several intrinsic factors, including organelles (e.g., centrioles and mitochondria) and molecules (e.g., proteins and RNAs), which are involved in important steps of reproductive biology such as spermatogenesis, sperm maturation, oocyte fertilization and embryo development. These factors constitute potential biomarkers of "viable sperm" and male fertility status and may become major assets for diagnosing instances of idiopathic male infertility in both humans and livestock animals. A better understanding of the mechanism of action of these sperm intrinsic factors in the regulation of reproductive and developmental processes still presents a major challenge that must be addressed. This review assembles the main data regarding morpho-functional and intrinsic sperm features that are associated with male infertility, with a particular focus on microRNA (miRNA) molecules.
Keywords: cattle; diagnosis; human; infertility; intrinsic factors; ncRNAs; semen; spermatozoa.
Copyright © 2020 Alves, Celeghini and Belleannée.
Figures


References
-
- Aarabi M., Balakier H., Bashar S., Moskovtsev S. I., Sutovsky P., Librach C. L., et al. (2014). Sperm content of postacrosomal WW binding protein is related to fertilization outcomes in patients undergoing assisted reproductive technology. Fertil. Steril. 102 440–447. 10.1016/j.fertnstert.2014.05.003 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

