SARS-CoV-2 Codon Usage Bias Downregulates Host Expressed Genes With Similar Codon Usage
- PMID: 32974353
- PMCID: PMC7468442
- DOI: 10.3389/fcell.2020.00831
SARS-CoV-2 Codon Usage Bias Downregulates Host Expressed Genes With Similar Codon Usage
Abstract
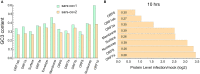
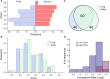
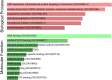
Severe acute respiratory syndrome has spread quickly throughout the world and was declared a pandemic by the World Health Organization (WHO). The pathogenic agent is a new coronavirus (SARS-CoV-2) that infects pulmonary cells with great effectiveness. In this study we focus on the codon composition for the viral protein synthesis and its relationship with the protein synthesis of the host. Our analysis reveals that SARS-CoV-2 preferred codons have poor representation of G or C nucleotides in the third position, a characteristic which could result in an unbalance in the tRNAs pools of the infected cells with serious implications in host protein synthesis. By integrating this observation with proteomic data from infected cells, we observe a reduced translation rate of host proteins associated with highly expressed genes and that they share the codon usage bias of the virus. The functional analysis of these genes suggests that this mechanism of epistasis can contribute to understanding how this virus evades the immune response and the etiology of some deleterious collateral effect as a result of the viral replication. In this manner, our finding contributes to the understanding of the SARS-CoV-2 pathogeny and could be useful for the design of a vaccine based on the live attenuated strategy.
Keywords: SARS-CoV-2; codon optimality; codon usage bias; pathogeny; translational control; vaccine design.
Copyright © 2020 Alonso and Diambra.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

