The Role of Machine Learning in Spine Surgery: The Future Is Now
- PMID: 32974382
- PMCID: PMC7472375
- DOI: 10.3389/fsurg.2020.00054
The Role of Machine Learning in Spine Surgery: The Future Is Now
Abstract
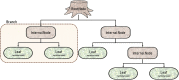
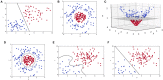
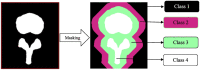
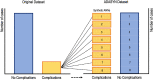
The recent influx of machine learning centered investigations in the spine surgery literature has led to increased enthusiasm as to the prospect of using artificial intelligence to create clinical decision support tools, optimize postoperative outcomes, and improve technologies used in the operating room. However, the methodology underlying machine learning in spine research is often overlooked as the subject matter is quite novel and may be foreign to practicing spine surgeons. Improper application of machine learning is a significant bioethics challenge, given the potential consequences of over- or underestimating the results of such studies for clinical decision-making processes. Proper peer review of these publications requires a baseline familiarity of the language associated with machine learning, and how it differs from classical statistical analyses. This narrative review first introduces the overall field of machine learning and its role in artificial intelligence, and defines basic terminology. In addition, common modalities for applying machine learning, including classification and regression decision trees, support vector machines, and artificial neural networks are examined in the context of examples gathered from the spine literature. Lastly, the ethical challenges associated with adapting machine learning for research related to patient care, as well as future perspectives on the potential use of machine learning in spine surgery, are discussed specifically.
Keywords: artificial intelligence; deep learning; machine learning; orthopedic surgery; spine surgery.
Copyright © 2020 Chang, Canseco, Nicholson, Patel and Vaccaro.
Figures







References
-
- Krzywinski M, Altman N. Classification and regression trees. Nat Methods. (2017) 14:757–8. 10.1038/nmeth.4370 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

