Middle East respiratory coronavirus (MERS-CoV) spike (S) protein vesicular stomatitis virus pseudoparticle neutralization assays offer a reliable alternative to the conventional neutralization assay in human seroepidemiological studies
- PMID: 32974558
- PMCID: PMC7472544
- DOI: 10.1099/acmi.0.000057
Middle East respiratory coronavirus (MERS-CoV) spike (S) protein vesicular stomatitis virus pseudoparticle neutralization assays offer a reliable alternative to the conventional neutralization assay in human seroepidemiological studies
Abstract
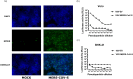
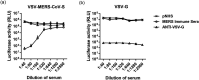
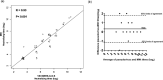
Middle East respiratory syndrome coronavirus (MERS-CoV) is a novel zoonotic coronavirus that was identified in 2012. MERS-CoV infection in humans can result in an acute, severe respiratory disease and in some cases multi-organ failure; the global mortality rate is approximately 35 %. The MERS-CoV spike (S) protein is a major target for neutralizing antibodies in infected patients. The MERS-CoV microneutralization test (MNt) is the gold standard method for demonstrating prior infection. However, this method requires the use of live MERS-CoV in biosafety level 3 (BSL-3) containment. The present work describes the generation and validation of S protein-bearing vesicular stomatitis virus (VSV) pseudotype particles (VSV-MERS-CoV-S) in which the VSV glycoprotein G gene has been replaced by the luciferase reporter gene, followed by the establishment of a pseudoparticle-based neutralization test to detect MERS-CoV neutralizing antibodies under BSL-2 conditions. Using a panel of human sera from confirmed MERS-CoV patients, the VSV-MERS-CoV particle neutralization assay produced results that were highly comparable to those of the microneutralization test using live MERS-CoV. The results suggest that the VSV-MERS-CoV-S pseudotype neutralization assay offers a highly specific, sensitive and safer alternative method to detect MERS-CoV neutralizing antibodies in human sera.
Keywords: Middle East respiratory syndrome coronavirus (MERS-CoV); luciferase; microneutralization test (MNt); neutralizing antibodies; pseudoparticle; vesicular stomatitis virus.
© 2019 The Authors.
Conflict of interest statement
The authors declare that there are no conflicts of interest. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Names of specific vendors, manufacturers, or products are included for public health and informational purposes; inclusion does not imply endorsement of the vendors, manufacturers, or products by the Centers for Disease Control and Prevention or the US Department of Health and Human Services.
Figures


Similar articles
-
Middle East Respiratory Syndrome Coronavirus Antibodies in Bactrian and Hybrid Camels from Dubai.mSphere. 2020 Jan 22;5(1):e00898-19. doi: 10.1128/mSphere.00898-19. mSphere. 2020. PMID: 31969478 Free PMC article.
-
Ultrapotent Human Neutralizing Antibody Repertoires Against Middle East Respiratory Syndrome Coronavirus From a Recovered Patient.J Infect Dis. 2018 Sep 8;218(8):1249-1260. doi: 10.1093/infdis/jiy311. J Infect Dis. 2018. PMID: 29846635 Free PMC article.
-
Inability of rat DPP4 to allow MERS-CoV infection revealed by using a VSV pseudotype bearing truncated MERS-CoV spike protein.Arch Virol. 2015 Sep;160(9):2293-300. doi: 10.1007/s00705-015-2506-z. Epub 2015 Jul 4. Arch Virol. 2015. PMID: 26138557 Free PMC article.
-
Prospects for a MERS-CoV spike vaccine.Expert Rev Vaccines. 2018 Aug;17(8):677-686. doi: 10.1080/14760584.2018.1506702. Epub 2018 Aug 9. Expert Rev Vaccines. 2018. PMID: 30058403 Free PMC article. Review.
-
Pseudotyped Vesicular Stomatitis Virus-Severe Acute Respiratory Syndrome-Coronavirus-2 Spike for the Study of Variants, Vaccines, and Therapeutics Against Coronavirus Disease 2019.Front Microbiol. 2022 Jan 14;12:817200. doi: 10.3389/fmicb.2021.817200. eCollection 2021. Front Microbiol. 2022. PMID: 35095820 Free PMC article. Review.
Cited by
-
Systematical assessment of the impact of single spike mutations of SARS-CoV-2 Omicron sub-variants on the neutralization capacity of post-vaccination sera.Front Immunol. 2023 Nov 10;14:1288794. doi: 10.3389/fimmu.2023.1288794. eCollection 2023. Front Immunol. 2023. PMID: 38022629 Free PMC article.
-
Current diagnostic approaches to detect two important betacoronaviruses: Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).Pathol Res Pract. 2021 Sep;225:153565. doi: 10.1016/j.prp.2021.153565. Epub 2021 Jul 24. Pathol Res Pract. 2021. PMID: 34333398 Free PMC article. Review.
-
BNT162b vaccines protect rhesus macaques from SARS-CoV-2.Nature. 2021 Apr;592(7853):283-289. doi: 10.1038/s41586-021-03275-y. Epub 2021 Feb 1. Nature. 2021. PMID: 33524990
-
Cross-Reactive Antibodies to SARS-CoV-2 and MERS-CoV in Pre-COVID-19 Blood Samples from Sierra Leoneans.Viruses. 2021 Nov 21;13(11):2325. doi: 10.3390/v13112325. Viruses. 2021. PMID: 34835131 Free PMC article.
-
Recent Developments in SARS-CoV-2 Neutralizing Antibody Detection Methods.Curr Med Sci. 2021 Dec;41(6):1052-1064. doi: 10.1007/s11596-021-2470-7. Epub 2021 Dec 21. Curr Med Sci. 2021. PMID: 34935114 Free PMC article. Review.
References
-
- Bermingham A, Chand MA, Brown CS, Aarons E, Tong C, et al. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the middle East, September 2012. Euro Surveill. 2012;17:20290. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
