Laboratory methods supporting measles surveillance in Queensland, Australia, 2010-2017
- PMID: 32974570
- PMCID: PMC7470308
- DOI: 10.1099/acmi.0.000093
Laboratory methods supporting measles surveillance in Queensland, Australia, 2010-2017
Abstract
Purpose: Australia was officially recognised as having eliminated endemic measles transmission in 2014. Maintaining laboratory support for surveillance of vaccine-preventable diseases, such as measles, is an essential component of reaching and maintaining transmission-free status.
Methodology: Real-time and conventional PCR-based tools were used to detect, differentiate from measles vaccine virus (MeVV), and sequence fragments of measles viruses (MeV) identified from specimens collected in Queensland. Specimens were mostly from travellers who had visited or returned to Queensland from international or interstate sites or been in contact with a case from either group.
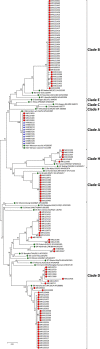
Results: Between 2010 and 2017, 13 678 specimens were tested in our laboratory using real-time RT-PCR (RT-rPCR), identifying 533 positives. Most specimens were swabs (70.98 %) and urines (25.56 %). A MeVV RT-rPCR was used on request and identified 154 instances of MeVV. MeV-positive extracts were genotyped as required. Genotypes identified among sequenced specimens included B3, D4, D8, D9, G3, and H1 as well as members of clade A as expected from the detection of MeV among virus introductions due to global travel and vaccination.
Conclusion: We describe the workflow employed and results from our laboratory between 2010 and 2017 for the sensitive detection of MeV infection, supporting high-quality surveillance to ensure the maintenance of Australia's measles-free status.
Keywords: Measles virus; diagnostics; epidemiology; measles.
© 2020 The Authors.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Measles Vaccine Virus RNA in Children More Than 100 Days after Vaccination.Viruses. 2019 Jul 10;11(7):636. doi: 10.3390/v11070636. Viruses. 2019. PMID: 31295941 Free PMC article.
-
What assay is optimal for the diagnosis of measles virus infection? An evaluation of the performance of a measles virus real-time reverse transcriptase PCR using the Cepheid SmartCycler(®) and antigen detection by immunofluorescence.J Clin Virol. 2015 Sep;70:46-52. doi: 10.1016/j.jcv.2015.07.004. Epub 2015 Jul 10. J Clin Virol. 2015. PMID: 26305819
-
Genetic Characterization of the H Gene of MeV Strains (H1, B3, and D4) Recently Circulated in Iran for Improving the Molecular Measles Surveillance in the National Measles Lab.Iran J Public Health. 2023 Aug;52(8):1730-1738. doi: 10.18502/ijph.v52i8.13412. Iran J Public Health. 2023. PMID: 37744531 Free PMC article.
-
Eradication of measles: remaining challenges.Med Microbiol Immunol. 2016 Jun;205(3):201-8. doi: 10.1007/s00430-016-0451-4. Epub 2016 Mar 2. Med Microbiol Immunol. 2016. PMID: 26935826 Free PMC article. Review.
-
Measles.Nat Rev Dis Primers. 2016 Jul 14;2:16049. doi: 10.1038/nrdp.2016.49. Nat Rev Dis Primers. 2016. PMID: 27411684 Review.
Cited by
-
Detection of measles vaccine virus and measles-specific immunoglobulin M in children vaccinated against measles-mumps-rubella during measles outbreak.Vaccine X. 2024 Apr 24;18:100491. doi: 10.1016/j.jvacx.2024.100491. eCollection 2024 Jun. Vaccine X. 2024. PMID: 38746062 Free PMC article.
-
Measles Vaccine Virus RNA in Children More Than 100 Days after Vaccination.Viruses. 2019 Jul 10;11(7):636. doi: 10.3390/v11070636. Viruses. 2019. PMID: 31295941 Free PMC article.
References
-
- Wang L-F, Collins PLF RAM, Kurath G, Lamb RA, Randall RE, et al. Paramyxoviridae: International Committee on taxonomy of viruses (ICTV)
Associated data
LinkOut - more resources
Full Text Sources
