Incorporating Real-time Influenza Detection Into the Test-negative Design for Estimating Influenza Vaccine Effectiveness: The Real-time Test-negative Design (rtTND)
- PMID: 32974644
- PMCID: PMC8096266
- DOI: 10.1093/cid/ciaa1453
Incorporating Real-time Influenza Detection Into the Test-negative Design for Estimating Influenza Vaccine Effectiveness: The Real-time Test-negative Design (rtTND)
Abstract
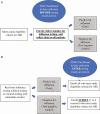
With rapid and accurate molecular influenza testing now widely available in clinical settings, influenza vaccine effectiveness (VE) studies can prospectively select participants for enrollment based on real-time results rather than enrolling all eligible patients regardless of influenza status, as in the traditional test-negative design (TND). Thus, we explore advantages and disadvantages of modifying the TND for estimating VE by using real-time, clinically available viral testing results paired with acute respiratory infection eligibility criteria for identifying influenza cases and test-negative controls prior to enrollment. This modification, which we have called the real-time test-negative design (rtTND), has the potential to improve influenza VE studies by optimizing the case-to-test-negative control ratio, more accurately classifying influenza status, improving study efficiency, reducing study cost, and increasing study power to adequately estimate VE. Important considerations for limiting biases in the rtTND include the need for comprehensive clinical influenza testing at study sites and accurate influenza tests.
Keywords: clinical testing; influenza; real-time test-negative design; test-negative design; vaccine effectiveness.
Published by Oxford University Press for the Infectious Diseases Society of America 2020.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

