Structural basis for DNA recognition and allosteric control of the retinoic acid receptors RAR-RXR
- PMID: 32974652
- PMCID: PMC7515732
- DOI: 10.1093/nar/gkaa697
Structural basis for DNA recognition and allosteric control of the retinoic acid receptors RAR-RXR
Abstract
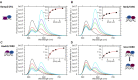
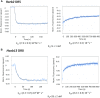
Retinoic acid receptors (RARs) as a functional heterodimer with retinoid X receptors (RXRs), bind a diverse series of RA-response elements (RAREs) in regulated genes. Among them, the non-canonical DR0 elements are bound by RXR-RAR with comparable affinities to DR5 elements but DR0 elements do not act transcriptionally as independent RAREs. In this work, we present structural insights for the recognition of DR5 and DR0 elements by RXR-RAR heterodimer using x-ray crystallography, small angle x-ray scattering, and hydrogen/deuterium exchange coupled to mass spectrometry. We solved the crystal structures of RXR-RAR DNA-binding domain in complex with the Rarb2 DR5 and RXR-RXR DNA-binding domain in complex with Hoxb13 DR0. While cooperative binding was observed on DR5, the two molecules bound non-cooperatively on DR0 on opposite sides of the DNA. In addition, our data unveil the structural organization and dynamics of the multi-domain RXR-RAR DNA complexes providing evidence for DNA-dependent allosteric communication between domains. Differential binding modes between DR0 and DR5 were observed leading to differences in conformation and structural dynamics of the multi-domain RXR-RAR DNA complexes. These results reveal that the topological organization of the RAR binding element confer regulatory information by modulating the overall topology and structural dynamics of the RXR-RAR heterodimers.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Chambon P. The nuclear receptor superfamily: a personal retrospect on the first two decades. Mol. Endocrinol. 2005; 19:1418–1428. - PubMed
-
- Benbrook D.M., Chambon P., Rochette-Egly C., Asson-Batres M.A.. History of retinoic acid receptors. Subcell. Biochem. 2014; 70:1–20. - PubMed
-
- di Masi A., Leboffe L., De Marinis E., Pagano F., Cicconi L., Rochette-Egly C., Lo-Coco F., Ascenzi P., Nervi C.. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol. Aspects Med. 2015; 41:1–115. - PubMed
-
- Gronemeyer H., Gustafsson J.A., Laudet V.. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 2004; 3:950–964. - PubMed
-
- Perissi V., Rosenfeld M.G.. Controlling nuclear receptors: the circular logic of cofactor cycles. Nat. Rev. Mol. Cell Biol. 2005; 6:542–554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

