Comparative analysis of dimethyl fumarate and fingolimod in relapsing-remitting multiple sclerosis
- PMID: 32974794
- PMCID: PMC7914245
- DOI: 10.1007/s00415-020-10226-6
Comparative analysis of dimethyl fumarate and fingolimod in relapsing-remitting multiple sclerosis
Abstract
Background: Dimethyl fumarate and fingolimod are oral disease modifying treatments (DMTs) that reduce relapse activity and slow disability worsening in relapsing-remitting multiple sclerosis (RRMS).
Objective: To compare the effectiveness of dimethyl fumarate and fingolimod in a real-world setting, where both agents are licensed as a first-line DMT for the treatment of RRMS.
Methods: We identified patients with RRMS commencing dimethyl fumarate or fingolimod in the Swiss Federation for Common Tasks of Health Insurances (SVK) Registry between August 2014 and July 2019. Propensity score-matching was applied to select subpopulations with comparable baseline characteristics. Relapses and disability outcomes were compared in paired, pairwise-censored analyses.
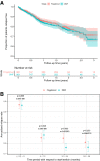
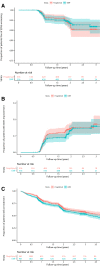
Results: Of the 2113 included patients, 1922 were matched (dimethyl fumarate, n = 961; fingolimod, n = 961). Relapse rates did not differ between the groups (incident rate ratio 1.0, 95%CI 0.8-1.2, p = 0.86). Moreover, no difference in the hazard of 1-year confirmed disability worsening (hazard ratio [HR] 0.9; 95%CI 0.6-1.6; p = 0.80) or disability improvement (HR 0.9; 95%CI 0.6-1.2; p = 0.40) was detected. These findings were consistent both for treatment-naïve patients and patients switching from another DMT.
Conclusion: Dimethyl fumarate and fingolimod have comparable effectiveness regarding reduction of relapses and disability worsening in RRMS.
Keywords: Dimethyl fumarate; Effectiveness; Fingolimod; Multiple sclerosis; Relapsing–remitting.
Conflict of interest statement
JL has received research support from Innosuisse-Swiss Innovation Agency, Biogen and Novartis and served on advisory boards for Roche and Teva. PB reports no conflicts of interest. CL reports no conflicts of interest. PH is an employee of the Swiss Federation for Common Tasks of Health Insurances. TD received speaker fees, research support, travel support, and/or served on Advisory Boards or Steering Committees of Novartis Pharma, Merck Serono, Biogen, Teva, Bayer-Schering, GeNeuro, Mitsubishi Pharma, MedDay, Roche, and Genzyme. JK’s institution received research support from Swiss MS Society, Biogen, Novartis, Roche, Genzyme, and Merck Serono; he received research support from Bayer AG, Genzyme, Novartis, Roche Pharma (Schweiz) AG, Swiss National Research Foundation, ECTRIMS, University of Basel, and Swiss MS Society. The University Hospital Basel, as the employer of L.K., has received and dedicated to research support fees for board membership, consultancy or speaking, or grants in the past 3 years from Actelion, Advancell, Allozyne, Bayer, Bayhill, Biogen Idec, BioMarin, CSL Behring, Eli Lilly EU, Genmab, GeNeuro SA, Gianni Rubatto Foundation, Glenmark, Merck Serono, MediciNova, Mitsubishi Pharma, Novartis, Novartis Research Foundation, Novo Nordisk, Peptimmune, Roche, Roche Research Foundation, Santhera, Sanofi-Aventis, Swiss MS Society, Swiss National Research Foundation, Teva Pharmaceutical Industries Ltd, UCB, and Wyeth. Ö.Y.’s institution (University Hospital Basel) received honoraria for lectures from Teva, Novartis and Bayer Schering exclusively used for funding of research or educational courses.
Figures
References
-
- Swissmedic (2017) Gilenya, Kapseln (Fingolimod), https://www.swissmedic.ch/zulassungen/00153/00189/00200/00927/index.html.... Accessed 16 Nov 2017
-
- Swissmedic (2017) Tecfidera, magensaftresistente Kapseln (Dimethylis fumaras)https://www.swissmedic.ch/zulassungen/00153/00189/00200/02374/index.html.... Accessed 16 Nov 2017
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

