Colonic delivery of vasoactive intestinal peptide nanomedicine alleviates colitis and shows promise as an oral capsule
- PMID: 32975467
- PMCID: PMC7713900
- DOI: 10.2217/nnm-2020-0280
Colonic delivery of vasoactive intestinal peptide nanomedicine alleviates colitis and shows promise as an oral capsule
Abstract
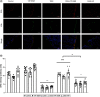
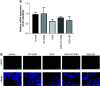
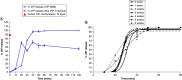
Aim: To evaluate the efficacy of locally delivered nanomedicine, vasoactive intestinal peptide in sterically stabilized micelles (VIP-SSM) to the colon and conduct in vitro release studies of a potential oral formulation. Materials & methods: Intracolonic instillation of VIP-SSM was tested in a mouse model of dextran sulfate sodium-induced colitis. Based on the effective mouse dose, human equivalent dose containing nanomedicine powder was filled into enteric coated capsules for in vitro release testing. Results: Colonic delivery of VIP-SSM significantly alleviated colitis. VIP-SSM containing capsules completely dissolved at colonic pH allowing micelles to reform with active VIP. Capsule formulations exhibited reproducible release profiles when stored up to 6 weeks demonstrating stability. Conclusion: VIP-SSM is an effective nanomedicine formulation which appears to have potential for oral treatment of colitis in humans. [Formula: see text].
Keywords: VIP nanomedicine; colitis; colonic delivery; inflammatory bowel disease; oral capsule form; oral nanomedicine; slc26a3; sterically stabilized micelles; targeted delivery.
Conflict of interest statement
These studies were supported by the NIDDK grants R01 DK54016, R01 DK92441 (PK Dudeja) and the Department of Veterans Affairs BX 002011 (PK Dudeja) and VA SRCS Award (IK6 BX005242, PK Dudeja), BX 002867 (S Saksena), BX004719 (A Kumar), UIC Dean’s Fellowship (D Jayawardena) and TUBITAK Award (H Onyuksel). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR15482 from the National Center for Research Resources, NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures





Similar articles
-
Vasoactive Intestinal Peptide Nanomedicine for the Management of Inflammatory Bowel Disease.Mol Pharm. 2017 Nov 6;14(11):3698-3708. doi: 10.1021/acs.molpharmaceut.7b00452. Epub 2017 Oct 19. Mol Pharm. 2017. PMID: 28991483 Free PMC article.
-
GLP-1 nanomedicine alleviates gut inflammation.Nanomedicine. 2017 Feb;13(2):659-665. doi: 10.1016/j.nano.2016.08.004. Epub 2016 Aug 20. Nanomedicine. 2017. PMID: 27553076 Free PMC article.
-
Targeting breast cancer using pirarubicin-loaded vasoactive intestinal peptide grafted sterically stabilized micelles.Eur J Pharm Sci. 2021 Jul 1;162:105830. doi: 10.1016/j.ejps.2021.105830. Epub 2021 Apr 2. Eur J Pharm Sci. 2021. PMID: 33819623
-
Novel, biocompatible, and disease modifying VIP nanomedicine for rheumatoid arthritis.Mol Pharm. 2013 Feb 4;10(2):728-38. doi: 10.1021/mp300539f. Epub 2013 Jan 23. Mol Pharm. 2013. PMID: 23211088 Free PMC article.
-
Colonic vasoactive intestinal polypeptide in ulcerative colitis.J Physiol Paris. 1993;87(5):307-11. doi: 10.1016/0928-4257(93)90037-t. J Physiol Paris. 1993. PMID: 8298608 Review.
Cited by
-
Oral Delivery of Biologics in Inflammatory Bowel Disease Treatment.Front Bioeng Biotechnol. 2021 Jun 3;9:675194. doi: 10.3389/fbioe.2021.675194. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34150733 Free PMC article. Review.
-
Emerging Nanotechnologies and Microbiome Engineering for the Treatment of Inflammatory Bowel Disease.Mol Pharm. 2022 Dec 5;19(12):4393-4410. doi: 10.1021/acs.molpharmaceut.2c00222. Epub 2022 Jul 25. Mol Pharm. 2022. PMID: 35878420 Free PMC article. Review.
-
Oral Sheep Milk-Derived Exosome Therapeutics for Cadmium-Induced Inflammatory Bowel Disease.Int J Mol Sci. 2025 Apr 2;26(7):3299. doi: 10.3390/ijms26073299. Int J Mol Sci. 2025. PMID: 40244136 Free PMC article.
References
-
- Abad C, Waschek JA. Immunomodulatory roles of VIP and PACAP in models of multiple sclerosis. Curr. Pharm. Des. 17(10), 1025–1035 (2011). - PubMed
-
- Delgado M, Martinez C, Pozo D. et al. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-α and IL-6. J. Immunol. 162(2), 1200–1205 (1999). - PubMed
-
- Gomariz R, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr. Pharm. Des. 7(2), 89–111 (2001). - PubMed
-
- Smalley S, Barrow P, Foster N. Immunomodulation of innate immune responses by vasoactive intestinal peptide (VIP): its therapeutic potential in inflammatory disease. Clin. Exp. Immunol. 157(2), 225–234 (2009). - PMC - PubMed
-
• Highlights the immunomodulatory properties of vasoactive intestinal peptide (VIP) and therefore forms the basis of its use in inflammatory diseases.
-
- Seo S, Miyake H, Alganabi M. et al. Vasoactive intestinal peptide decreases inflammation and tight junction disruption in experimental necrotizing enterocolitis. J. Pediatr. Surg. 54(12), 2520–2523 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
