Neuraminidase-1 promotes heart failure after ischemia/reperfusion injury by affecting cardiomyocytes and invading monocytes/macrophages
- PMID: 32975669
- PMCID: PMC7519006
- DOI: 10.1007/s00395-020-00821-z
Neuraminidase-1 promotes heart failure after ischemia/reperfusion injury by affecting cardiomyocytes and invading monocytes/macrophages
Abstract
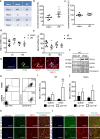
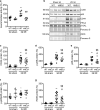
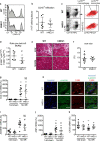
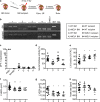
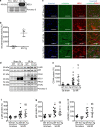
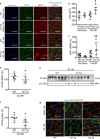
Neuraminidase (NEU)1 forms a multienzyme complex with beta-galactosidase (β-GAL) and protective-protein/cathepsin (PPC) A, which cleaves sialic-acids from cell surface glycoconjugates. We investigated the role of NEU1 in the myocardium after ischemia/reperfusion (I/R). Three days after inducing I/R, left ventricles (LV) of male mice (3 months-old) displayed upregulated neuraminidase activity and increased NEU1, β-GAL and PPCA expression. Mice hypomorphic for neu1 (hNEU1) had less neuraminidase activity, fewer pro-inflammatory (Lin-CD11b+F4/80+Ly-6Chigh), and more anti-inflammatory macrophages (Lin-CD11b+F4/80+Ly-6Clow) 3 days after I/R, and less LV dysfunction 14 days after I/R. WT mice transplanted with hNEU1-bone marrow (BM) and hNEU1 mice with WT-BM showed significantly better LV function 14 days after I/R compared with WT mice with WT-BM. Mice with a cardiomyocyte-specific NEU1 overexpression displayed no difference in inflammation 3 days after I/R, but showed increased cardiomyocyte hypertrophy, reduced expression and mislocalization of Connexin-43 in gap junctions, and LV dysfunction despite a similar infarct scar size to WT mice 14 days after I/R. The upregulation of NEU1 after I/R contributes to heart failure by promoting inflammation in invading monocytes/macrophages, enhancing cardiomyocyte hypertrophy, and impairing gap junction function, suggesting that systemic NEU1 inhibition may reduce heart failure after I/R.
Keywords: Inflammation; Ischemia/reperfusion; Monocytes; Neuraminidase 1; Sialidase 1.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Overexpression of A kinase interacting protein 1 attenuates myocardial ischaemia/reperfusion injury but does not influence heart failure development.Cardiovasc Res. 2016 Aug 1;111(3):217-26. doi: 10.1093/cvr/cvw161. Epub 2016 Jun 14. Cardiovasc Res. 2016. PMID: 27302402
-
G protein-coupled receptor kinase 5 (GRK5) contributes to impaired cardiac function and immune cell recruitment in post-ischemic heart failure.Cardiovasc Res. 2022 Jan 7;118(1):169-183. doi: 10.1093/cvr/cvab044. Cardiovasc Res. 2022. PMID: 33560342 Free PMC article.
-
Splenic Ly6Chi monocytes contribute to adverse late post-ischemic left ventricular remodeling in heme oxygenase-1 deficient mice.Basic Res Cardiol. 2017 Jul;112(4):39. doi: 10.1007/s00395-017-0629-y. Epub 2017 May 22. Basic Res Cardiol. 2017. PMID: 28534119 Free PMC article.
-
Immune cells as targets for cardioprotection: new players and novel therapeutic opportunities.Cardiovasc Res. 2019 Jun 1;115(7):1117-1130. doi: 10.1093/cvr/cvz050. Cardiovasc Res. 2019. PMID: 30825305 Free PMC article. Review.
-
Lysosomal multienzyme complex: pros and cons of working together.Cell Mol Life Sci. 2014 Jun;71(11):2017-32. doi: 10.1007/s00018-013-1538-3. Epub 2013 Dec 15. Cell Mol Life Sci. 2014. PMID: 24337808 Free PMC article. Review.
Cited by
-
Changes in lung sialidases in male and female mice after bleomycin aspiration.Exp Lung Res. 2022 Nov-Dec;48(9-10):291-304. doi: 10.1080/01902148.2022.2144548. Epub 2022 Nov 16. Exp Lung Res. 2022. PMID: 36382835 Free PMC article.
-
Melatonin Attenuates Ischemia/Reperfusion-Induced Oxidative Stress by Activating Mitochondrial Fusion in Cardiomyocytes.Oxid Med Cell Longev. 2022 Jan 10;2022:7105181. doi: 10.1155/2022/7105181. eCollection 2022. Oxid Med Cell Longev. 2022. Retraction in: Oxid Med Cell Longev. 2023 Oct 11;2023:9815786. doi: 10.1155/2023/9815786. PMID: 35047108 Free PMC article. Retracted.
-
Biological function of sialic acid and sialylation in human health and disease.Cell Death Discov. 2024 Sep 30;10(1):415. doi: 10.1038/s41420-024-02180-3. Cell Death Discov. 2024. PMID: 39349440 Free PMC article. Review.
-
Protective Effect of Nicorandil on Cardiac Microvascular Injury: Role of Mitochondrial Integrity.Oxid Med Cell Longev. 2021 Jul 3;2021:4665632. doi: 10.1155/2021/4665632. eCollection 2021. Oxid Med Cell Longev. 2021. Retraction in: Oxid Med Cell Longev. 2023 Oct 11;2023:9845162. doi: 10.1155/2023/9845162. PMID: 34285763 Free PMC article. Retracted. Review.
-
Combined Pharmacological Modulation of Translational and Transcriptional Activity Signaling Pathways as a Promising Therapeutic Approach in Children with Myocardial Changes.Biomolecules. 2024 Apr 13;14(4):477. doi: 10.3390/biom14040477. Biomolecules. 2024. PMID: 38672493 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

