Lentinan improved the efficacy of vaccine against Trichinella spiralis in an NLRP3 dependent manner
- PMID: 32976511
- PMCID: PMC7518624
- DOI: 10.1371/journal.pntd.0008632
Lentinan improved the efficacy of vaccine against Trichinella spiralis in an NLRP3 dependent manner
Abstract
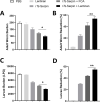
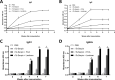
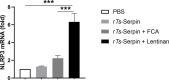
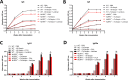
There is an urgent need for the development of new, improved vaccine adjuvants against T. spiralis infection. Polysaccharides are effective, safe, and biodegradable as adjuvant. In our study, we first observed the protective efficacy of lentinan as adjuvant against helminth T. spiralis infection. Recombinant T. spiralis Serpin (rTs-Serpin) immunoscreened from a cDNA library of T. spiralis, as a vaccine, protect host against Trichinella infection. The reduction rate of helminth burden of rTs-Serpin+lentinan-immunized mice was significantly increased compared with rTs-Serpin+FCA -immunized mice. rTs-Serpin+lentinan induced IgG1-dominant immune response and higher levels of IFN-γ and IL-4. rTs-Serpin+lentinan displayed a lower reduction rate of parasite burden in NLRP3-/- mice than that in WT mice and lower level of IgG1 than that in WT mice. The level of IL-4, but not IFN-γ, from NLRP3-/- mice immunized by rTs-Serpin+lentinan was significantly lower than that from WT mice, suggesting that NLRP3 is associated with rTs-Serpin+lentinan -triggering Th2 protective immunity against T. spiralis infection. In summary, we revealed that lentinan was a novel adjuvant against T. spiralis infection via NLRP3. NLRP3 therefore represents an important target for adjuvant discovery and the control of T. spiralis infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


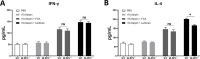
Similar articles
-
Influence of adjuvant formulation on inducing immune response in mice immunized with a recombinant serpin from Trichinella spiralis.Parasite Immunol. 2017 Jul;39(7). doi: 10.1111/pim.12437. Epub 2017 May 15. Parasite Immunol. 2017. PMID: 28445612
-
Vaccination of mice with an antigenic serine protease-like protein elicits a protective immune response against Trichinella spiralis infection.J Parasitol. 2013 Jun;99(3):426-32. doi: 10.1645/12-46.1. Epub 2012 Dec 19. J Parasitol. 2013. PMID: 23252743
-
Partially Protective Immunity Induced by a 20 kDa Protein Secreted by Trichinella spiralis Stichocytes.PLoS One. 2015 Aug 19;10(8):e0136189. doi: 10.1371/journal.pone.0136189. eCollection 2015. PLoS One. 2015. PMID: 26288365 Free PMC article.
-
Vaccines against Trichinella spiralis: Progress, challenges and future prospects.Transbound Emerg Dis. 2018 Dec;65(6):1447-1458. doi: 10.1111/tbed.12917. Epub 2018 Jun 6. Transbound Emerg Dis. 2018. PMID: 29873198 Review.
-
Induction of protection in murine experimental models against Trichinella spiralis: an up-to-date review.J Helminthol. 2015 Sep;89(5):526-39. doi: 10.1017/S0022149X15000140. Epub 2015 Mar 12. J Helminthol. 2015. PMID: 25761655 Review.
Cited by
-
Interaction between Intestinal Parasites and the Gut Microbiota: Implications for the Intestinal Immune Response and Host Defence.Pathogens. 2024 Jul 23;13(8):608. doi: 10.3390/pathogens13080608. Pathogens. 2024. PMID: 39204209 Free PMC article. Review.
-
Progress and prospect of polysaccharides as adjuvants in vaccine development.Virulence. 2024 Dec;15(1):2435373. doi: 10.1080/21505594.2024.2435373. Epub 2024 Dec 5. Virulence. 2024. PMID: 39601191 Free PMC article. Review.
-
Clostridium butyricum and Bifidobacterium pseudolongum Attenuate the Development of Cardiac Fibrosis in Mice.Microbiol Spectr. 2022 Dec 21;10(6):e0252422. doi: 10.1128/spectrum.02524-22. Epub 2022 Nov 1. Microbiol Spectr. 2022. PMID: 36318049 Free PMC article.
-
Lentinan -triggered butyrate-producing bacteria drive the expulsion of the intestinal helminth Trichinella spiralis in mice.Front Immunol. 2022 Jul 28;13:926765. doi: 10.3389/fimmu.2022.926765. eCollection 2022. Front Immunol. 2022. PMID: 35967395 Free PMC article.
-
Oral vaccination with recombinant Lactobacillus plantarum encoding Trichinella spiralis inorganic pyrophosphatase elicited a protective immunity in BALB/c mice.PLoS Negl Trop Dis. 2021 Oct 26;15(10):e0009865. doi: 10.1371/journal.pntd.0009865. eCollection 2021 Oct. PLoS Negl Trop Dis. 2021. PMID: 34699522 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

