NH2 -terminal fragment of ZF21 protein suppresses tumor invasion via inhibiting the interaction of ZF21 with FAK
- PMID: 32976654
- PMCID: PMC7734166
- DOI: 10.1111/cas.14665
NH2 -terminal fragment of ZF21 protein suppresses tumor invasion via inhibiting the interaction of ZF21 with FAK
Abstract
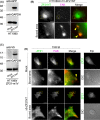
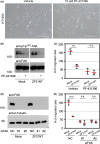
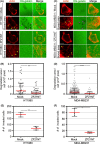
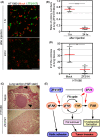
Cellular migration, coupled with the degradation of the extracellular matrix (ECM), is a key step in tumor invasion and represents a promising therapeutic target in malignant tumors. Focal adhesions (FAs) and invadopodia, which are distinct actin-based cellular structures, play key roles in cellular migration and ECM degradation, respectively. The molecular machinery coordinating the dynamics between FAs and invadopodia is not fully understood, although several lines of evidence suggest that the disassembly of FAs is an important step in triggering the formation of invadopodia. In a previous study, we identified the ZF21 protein as a regulator of both FA turnover and invadopodia-dependent ECM degradation. ZF21 interacts with multiple factors for FA turnover, including focal adhesion kinase (FAK), microtubules, m-Calpain, and Src homology region 2-containing protein tyrosine phosphatase 2 (SHP-2). In particular, the dephosphorylation of FAK by ZF21 is a key event in tumor invasion. However, the precise role of ZF21 binding to FAK remains unclear. We established a method to disrupt the interaction between ZF21 and FAK using the FAK-binding NH2 -terminal region of ZF21. Tumor cells expressing the ZF21-derived polypeptide had significantly decreased FA turnover, migration, invadopodia-dependent ECM degradation, and Matrigel invasion. Furthermore, the expression of the polypeptide inhibited an early step of experimental lung metastasis in mice. These findings indicate that the interaction of ZF21 with FAK is necessary for FA turnover as well as ECM degradation at the invadopodia. Thus, ZF21 is a potential regulator that coordinates the equilibrium between FA turnover and invadopodia activity by interacting with FAK.
Keywords: ZF21 protein; extracellular matrix; focal adhesion kinase; invadopodia; tumor metastasis.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no conflict of interest.
Figures



References
-
- Friedl P, Wolf K. Tumour‐cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362‐374. - PubMed
-
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679‐695. - PubMed
-
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895‐904. - PubMed
-
- Wehrle‐Haller B, Imhof B. The inner lives of focal adhesions. Trends Cell Biol. 2002;12:382‐389. - PubMed
-
- Zamir E, Geiger B. Molecular complexity and dynamics of cell‐matrix adhesions. J Cell Sci. 2001;114:3583‐3590. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

