Prognosis of patients with adult T-cell leukemia/lymphoma in Japan: A nationwide hospital-based study
- PMID: 32976684
- PMCID: PMC7734015
- DOI: 10.1111/cas.14658
Prognosis of patients with adult T-cell leukemia/lymphoma in Japan: A nationwide hospital-based study
Abstract
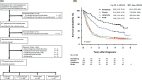
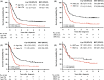
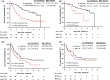
Adult T-cell leukemia/lymphoma (ATL) is a mature T-cell neoplasm and is classified into four subtypes (acute, lymphoma, chronic, and smoldering) according to the Shimoyama classification, established in 1991 through several nationwide surveys based on the clinical diversity of patients diagnosed in 1983-1987 in Japan. Thereafter, no such studies have been conducted. Recently, we conducted a nationwide hospital survey using the method of the 1980s studies, collected baseline data on 996 ATL patients diagnosed in 2010-2011 from 126 hospitals, and reported their unique epidemiological characteristics. Here, we report the follow-up results of registered ATL patients with the goal of evaluating current prognoses and treatment modalities as of 2016-2017. Of 770 evaluable patients, 391 (50.8%) had acute-type, 192 (24.9%) had lymphoma-type, 106 (13.8%) had chronic-type, and 81 (10.5%) had smoldering-type ATL. The initial therapy regimens used for acute/lymphoma-type ATL were vincristine, cyclophosphamide, doxorubicin and prednisone, followed by doxorubicin, ranimustine, and prednisone and then by vindesine, etoposide, carboplatin, and prednisone (VCAP-AMP-VECP)-like in 38.5/41.7% and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)-like in 14.6/13.7% of patients. Allogeneic hematopoietic stem cell transplantation was used to treat 15.9/10.4% of acute/lymphoma-type ATL patients. The 4-year survival rates (the median survival time, days) for acute-, lymphoma-, unfavorable chronic-, favorable chronic-, and smoldering-type ATL were 16.8% (252), 19.6% (305), 26.6% (572), 62.1% (1937), and 59.8% (1851), respectively. The 4-year survival rates for acute- and lymphoma-type ATL improved compared with those reported in 1991, but those for chronic- and smoldering-type ATL were not. Further efforts are warranted to develop more efficient therapeutic strategies to improve the prognosis of ATL in Japan.
Keywords: ATL; HTLV-1; Japanese nationwide survey; clinical subtypes; prognosis.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Kisato Nosaka reports personal fees from Celgene KK; Kenji Ishitsuka reports grants from Kyowa Hakko Kirin Co., Ltd. and personal fees from Celgene KK and Kyowa Hakko Kirin Co., Ltd.; Kenichi Ishizawa reports grants from Novartis, Abbie, Bayer, and SymBio, and personal fees from Takeda, Celgene KK, Novartis, Ono Pharmaceutical, Chugai Pharma, and Eizai; Takashi Ishida reports grants from Kyowa Hakko Kirin Co., Ltd., Bayer AG, and Celgene KK and personal fees from Kyowa Hakko Kirin Co., Ltd., Celgene KK, and Mundipharma KK; Atae Utsunomiya reports personal fees from Kyowa Hakko Kirin Co., Ltd and Celgene KK; Kensei Tobinai reports grants from Chugai Pharma, Kyowa Hakko Kirin Co., Ltd., Ono Pharmaceutical, Celgene KK, Janssen, Eisai, Mundi Pharma, Takeda, and Abbvie and personal fees from Eisai, Takeda, Mundipharma, HUYA Bioscience International, Kyowa Hakko Kirin Co., Ltd., Celgene KK, Chugai Pharma, Ono Pharmaceutical, Yakult, Daiichi Sankyo, Bristol‐Myers Squibb, Meiji Seika Kaisha, Solasia Pharma, Verastem, and Zenyaku Kogyo; Toshiki Watanabe reports grants from Solasia Pharma; Kunihiro Tsukasaki reports scholarship from Chugai Pharma. All the remaining authors have declared no conflicts of interest.
Figures

References
-
- Uchiyama T, Yodoi J, Sagawa K, et al. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481‐492. - PubMed
-
- Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609‐e616. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- H23-GanRinsho-Ippan-020/Grants-in-Aid from the Ministry of Health, Labour and Welfare of Japan
- H26-GanSeisaku-Ippan-006/Grants-in-Aid from the Ministry of Health, Labour and Welfare of Japan
- 17ck0106338h0001/the Japan Agency for Medical Research and Development
- 18ck0106338s0502/the Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources
Research Materials

