Insulin and Leptin/Upd2 Exert Opposing Influences on Synapse Number in Fat-Sensing Neurons
- PMID: 32976758
- PMCID: PMC7642105
- DOI: 10.1016/j.cmet.2020.08.017
Insulin and Leptin/Upd2 Exert Opposing Influences on Synapse Number in Fat-Sensing Neurons
Abstract
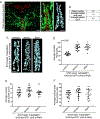
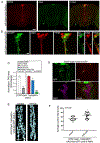
Energy-sensing neural circuits decide to expend or conserve resources based, in part, on the tonic, steady-state, energy-store information they receive. Tonic signals, in the form of adipose tissue-derived adipokines, set the baseline level of activity in the energy-sensing neurons, thereby providing context for interpretation of additional inputs. However, the mechanism by which tonic adipokine information establishes steady-state neuronal function has heretofore been unclear. We show here that under conditions of nutrient surplus, Upd2, a Drosophila leptin ortholog, regulates actin-based synapse reorganization to reduce bouton number in an inhibitory circuit, thus establishing a neural tone that is permissive for insulin release. Unexpectedly, we found that insulin feeds back on these same inhibitory neurons to conversely increase bouton number, resulting in maintenance of negative tone. Our results point to a mechanism by which two surplus-sensing hormonal systems, Upd2/leptin and insulin, converge on a neuronal circuit with opposing outcomes to establish energy-store-dependent neuron activity.
Keywords: Drosophila; JAK-STAT; Upd2; actin; arouser; basigin; energy homeostasis; gelsolin; inhibitory tone; insulin; leptin.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





Similar articles
-
Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion.Cell. 2012 Sep 28;151(1):123-37. doi: 10.1016/j.cell.2012.08.019. Cell. 2012. PMID: 23021220 Free PMC article.
-
Fat Body p53 Regulates Systemic Insulin Signaling and Autophagy under Nutrient Stress via Drosophila Upd2 Repression.Cell Rep. 2020 Oct 27;33(4):108321. doi: 10.1016/j.celrep.2020.108321. Cell Rep. 2020. PMID: 33113367 Free PMC article.
-
A Mechanism Coupling Systemic Energy Sensing to Adipokine Secretion.Dev Cell. 2017 Oct 9;43(1):83-98.e6. doi: 10.1016/j.devcel.2017.09.007. Dev Cell. 2017. PMID: 29017032 Free PMC article.
-
Inter-organ communication in Drosophila: Lipoproteins, adipokines, and immune-metabolic coordination.Curr Opin Cell Biol. 2025 Jun;94:102508. doi: 10.1016/j.ceb.2025.102508. Epub 2025 Apr 4. Curr Opin Cell Biol. 2025. PMID: 40187050 Review.
-
Hypothalamic carnitine metabolism integrates nutrient and hormonal feedback to regulate energy homeostasis.Mol Cell Endocrinol. 2015 Dec 15;418 Pt 1:9-16. doi: 10.1016/j.mce.2015.08.002. Epub 2015 Aug 8. Mol Cell Endocrinol. 2015. PMID: 26261054 Review.
Cited by
-
Thymosin Beta 15 Alters the Spatial Development of Thymic Epithelial Cells.Cells. 2022 Nov 19;11(22):3679. doi: 10.3390/cells11223679. Cells. 2022. PMID: 36429107 Free PMC article.
-
Adipocyte-Derived CCHamide-1, Eiger, Growth-Blocking Peptide 3, and Unpaired 2 Regulate Drosophila melanogaster Oogenesis.Biomolecules. 2025 Apr 1;15(4):513. doi: 10.3390/biom15040513. Biomolecules. 2025. PMID: 40305230 Free PMC article.
-
Adipocyte metabolic state regulates glial phagocytic function.bioRxiv [Preprint]. 2024 Sep 24:2024.09.24.614765. doi: 10.1101/2024.09.24.614765. bioRxiv. 2024. Update in: Cell Rep. 2025 May 27;44(5):115704. doi: 10.1016/j.celrep.2025.115704. PMID: 39386724 Free PMC article. Updated. Preprint.
-
S-9-PAHSA Protects Against High-Fat Diet-Induced Diabetes-Associated Cognitive Impairment via Gut Microbiota Regulation.CNS Neurosci Ther. 2025 May;31(5):e70417. doi: 10.1111/cns.70417. CNS Neurosci Ther. 2025. PMID: 40325622 Free PMC article.
-
Balancing energy expenditure and storage with growth and biosynthesis during Drosophila development.Dev Biol. 2021 Jul;475:234-244. doi: 10.1016/j.ydbio.2021.01.019. Epub 2021 Feb 11. Dev Biol. 2021. PMID: 33582116 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

