Novel Mechanistic PBPK Model to Predict Renal Clearance in Varying Stages of CKD by Incorporating Tubular Adaptation and Dynamic Passive Reabsorption
- PMID: 32977369
- PMCID: PMC7577018
- DOI: 10.1002/psp4.12553
Novel Mechanistic PBPK Model to Predict Renal Clearance in Varying Stages of CKD by Incorporating Tubular Adaptation and Dynamic Passive Reabsorption
Abstract
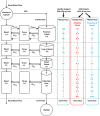
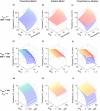
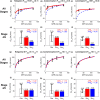
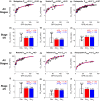
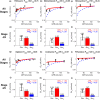
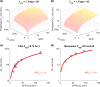
Chronic kidney disease (CKD) has significant effects on renal clearance (CLr ) of drugs. Physiologically-based pharmacokinetic (PBPK) models have been used to predict CKD effects on transporter-mediated renal active secretion and CLr for hydrophilic nonpermeable compounds. However, no studies have shown systematic PBPK modeling of renal passive reabsorption or CLr for hydrophobic permeable drugs in CKD. The goal of this study was to expand our previously developed and verified mechanistic kidney model to develop a universal model to predict changes in CLr in CKD for permeable and nonpermeable drugs that accounts for the dramatic nonlinear effect of CKD on renal passive reabsorption of permeable drugs. The developed model incorporates physiologically-based tubular changes of reduced water reabsorption/increased tubular flow rate per remaining functional nephron in CKD. The final adaptive kidney model successfully (absolute fold error (AFE) all < 2) predicted renal passive reabsorption and CLr for 20 permeable and nonpermeable test compounds across the stages of CKD. In contrast, use of proportional glomerular filtration rate reduction approach without addressing tubular adaptation processes in CKD to predict CLr generated unacceptable CLr predictions (AFE = 2.61-7.35) for permeable compounds in severe CKD. Finally, the adaptive kidney model accurately predicted CLr of para-amino-hippuric acid and memantine, two secreted compounds, in CKD, suggesting successful integration of active secretion into the model, along with passive reabsorption. In conclusion, the developed adaptive kidney model enables mechanistic predictions of in vivo CLr through CKD progression without any empirical scaling factors and can be used for CLr predictions prior to assessment of drug disposition in renal impairment.
© 2020 The Authors. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Both authors declared no competing interests for this work.
Figures
References
-
- Levey, A.S. & Coresh, J. Chronic kidney disease. Lancet 379, 165–180 (2012). - PubMed
-
- Zoccali, C. et al The systemic nature of CKD. Nat. Rev. Nephrol. 13, 344–358 (2017). - PubMed
-
- Bricker, N.S. , Morrin, P.A.F. & Kime, S.W. The pathologic physiology of chronic Bright’s disease. Am. J. Med. 28, 77–98 (1960). - PubMed
-
- KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 3, 19–62 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

