Interleukin-1β Alters Hebbian Synaptic Plasticity in Multiple Sclerosis
- PMID: 32977401
- PMCID: PMC7584038
- DOI: 10.3390/ijms21196982
Interleukin-1β Alters Hebbian Synaptic Plasticity in Multiple Sclerosis
Abstract
In multiple sclerosis (MS), inflammation alters synaptic transmission and plasticity, negatively influencing the disease course. In the present study, we aimed to explore the influence of the proinflammatory cytokine IL-1β on peculiar features of associative Hebbian synaptic plasticity, such as input specificity, using the paired associative stimulation (PAS). In 33 relapsing remitting-MS patients and 15 healthy controls, PAS was performed on the abductor pollicis brevis (APB) muscle. The effects over the motor hot spot of the APB and abductor digiti minimi (ADM) muscles were tested immediately after PAS and 15 and 30 min later. Intracortical excitability was tested with paired-pulse transcranial magnetic stimulation (TMS). The cerebrospinal fluid (CSF) levels of IL-1β were calculated. In MS patients, PAS failed to induce long-term potentiation (LTP)-like effects in the APB muscle and elicited a paradoxical motor-evoked potential (MEP) increase in the ADM. IL-1β levels were negatively correlated with the LTP-like response in the APB muscle. Moreover, IL-1β levels were associated with synaptic hyperexcitability tested with paired-pulse TMS. Synaptic hyperexcitability caused by IL-1β may critically contribute to alter Hebbian plasticity in MS, inducing a loss of topographic specificity.
Keywords: interleukin (IL)-1β; long-term potentiation (LTP); multiple sclerosis (MS); paired associative stimulation (PAS); synaptic plasticity; transcranial magnetic stimulation (TMS).
Conflict of interest statement
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Fabio Buttari acted as Advisory Board members of Teva and Roche and received honoraria for speaking or consultation fees from Merck Serono, Teva, Biogen Idec, Sanofi, and Novartis and non-financial support from Merck Serono, Teva, Biogen Idec, and Sanofi. Girolama Alessandra Marfia received honoraria for speaking, consultation fees, and travel funding from Roche, Almirall, Bayer Schering, Biogen Idec, Merck Serono, Novartis, Sanofi-Genzyme, Mylan, and Teva. She is the principal investigator in clinical trials for Actelion, Biogen Idec, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, Teva. Roberto Furlan has received honoraria as a speaker or for research support from Biogen, Novartis, Merck, Roche, Genzyme. Diego Centonze is an Advisory Board member of Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva and received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva. He is also the principal investigator in clinical trials for Bayer Schering, Biogen, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, and Teva. His preclinical and clinical research was supported by grants from Bayer Schering, Biogen Idec, Celgene, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Mario Stampanoni Bassi, Carolina Gabri Nicoletti, Francesco Mori, Luana Gilio, Ilaria Simonelli, Nicla De Paolis, Annamaria Finardi, Ennio Iezzi declare no conflict of interest.
Figures
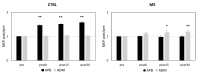
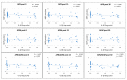


References
Publication types
MeSH terms
Substances
Grants and funding
- 2019/S/1 to DC/Fondazione Italiana Sclerosi Multipla
- Ricerca corrente-IRCCS Neuromed to DC/Ministero della Salute
- Ricerca Finalizzata 2018, RF-2018-12366144 to DC/Ministero della Salute
- Ricerca Finalizzata 2018, GR-2018-12366154 to FB/Ministero della Salute
- 5 × 1000 grant to IRCCS Neuromed./undefined <span style="color:gray;font-size:10px;">undefined</span>
LinkOut - more resources
Full Text Sources

