Bone Microenvironment and Osteosarcoma Metastasis
- PMID: 32977425
- PMCID: PMC7582690
- DOI: 10.3390/ijms21196985
Bone Microenvironment and Osteosarcoma Metastasis
Abstract
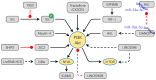
The bone microenvironment is an ideal fertile soil for both primary and secondary tumors to seed. The occurrence and development of osteosarcoma, as a primary bone tumor, is closely related to the bone microenvironment. Especially, the metastasis of osteosarcoma is the remaining challenge of therapy and poor prognosis. Increasing evidence focuses on the relationship between the bone microenvironment and osteosarcoma metastasis. Many elements exist in the bone microenvironment, such as acids, hypoxia, and chemokines, which have been verified to affect the progression and malignance of osteosarcoma through various signaling pathways. We thoroughly summarized all these regulators in the bone microenvironment and the transmission cascades, accordingly, attempting to furnish hints for inhibiting osteosarcoma metastasis via the amelioration of the bone microenvironment. In addition, analysis of the cross-talk between the bone microenvironment and osteosarcoma will help us to deeply understand the development of osteosarcoma. The cellular and molecular protagonists presented in the bone microenvironment promoting osteosarcoma metastasis will accelerate the exploration of novel therapeutic strategies towards osteosarcoma.
Keywords: bone microenvironment; metastasis; osteosarcoma; primary bone tumor; signal pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

