Unsaturated and Benzannulated N-Heterocyclic Carbene Complexes of Titanium and Hafnium: Impact on Catalysts Structure and Performance in Copolymerization of Cyclohexene Oxide with CO2
- PMID: 32977466
- PMCID: PMC7582562
- DOI: 10.3390/molecules25194364
Unsaturated and Benzannulated N-Heterocyclic Carbene Complexes of Titanium and Hafnium: Impact on Catalysts Structure and Performance in Copolymerization of Cyclohexene Oxide with CO2
Abstract
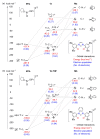
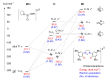
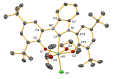
Tridentate, bis-phenolate N-heterocyclic carbenes (NHCs) are among the ligands giving the most selective and active group 4-based catalysts for the copolymerization of cyclohexene oxide (CHO) with CO2. In particular, ligands based on imidazolidin-2-ylidene (saturated NHC) moieties have given catalysts which exclusively form polycarbonate in moderate-to-high yields even under low CO2 pressure and at low copolymerization temperatures. Here, to evaluate the influence of the NHC moiety on the molecular structure of the catalyst and its performance in copolymerization, we extend this chemistry by synthesizing and characterizing titanium complexes bearing tridentate bis-phenolate imidazol-2-ylidene (unsaturated NHC) and benzimidazol-2-ylidene (benzannulated NHC) ligands. The electronic properties of the ligands and the nature of their bonds to titanium are studied using density functional theory (DFT) and natural bond orbital (NBO) analysis. The metal-NHC bond distances and bond strengths are governed by ligand-to-metal σ- and π-donation, whereas back-donation directly from the metal to the NHC ligand seems to be less important. The NHC π-acceptor orbitals are still involved in bonding, as they interact with THF and isopropoxide oxygen lone-pair donor orbitals. The new complexes are, when combined with [PPN]Cl co-catalyst, selective in polycarbonate formation. The highest activity, albeit lower than that of the previously reported Ti catalysts based on saturated NHC, was obtained with the benzannulated NHC-Ti catalyst. Attempts to synthesize unsaturated and benzannulated NHC analogues based on Hf invariably led, as in earlier work with Zr, to a mixture of products that include zwitterionic and homoleptic complexes. However, the benzannulated NHC-Hf complexes were obtained as the major products, allowing for isolation. Although these complexes selectively form polycarbonate, their catalytic performance is inferior to that of analogues based on saturated NHC.
Keywords: N-heterocyclic carbene; copolymerization of epoxide with CO2; density functional theory; hafnium; natural bond orbitals; titanium.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
N‑Heterocyclic Carbene-Based Group 4 Catalysts for the Terpolymerization of Cyclohexene Oxide and Cyclic Anhydrides with CO2.ACS Org Inorg Au. 2025 May 13;5(3):171-180. doi: 10.1021/acsorginorgau.5c00002. eCollection 2025 Jun 4. ACS Org Inorg Au. 2025. PMID: 40487033 Free PMC article.
-
Coordination behavior of bis-phenolate saturated and unsaturated N-heterocyclic carbene ligands to zirconium: reactivity and activity in the copolymerization of cyclohexene oxide with CO2.Dalton Trans. 2017 Jun 27;46(25):8065-8076. doi: 10.1039/c7dt01117b. Dalton Trans. 2017. PMID: 28604887
-
Group 4 metal complexes with new chiral pincer NHC-ligands: synthesis, structure and catalytic activity.Dalton Trans. 2014 Jun 14;43(22):8261-72. doi: 10.1039/c4dt00510d. Dalton Trans. 2014. PMID: 24710509
-
Recent advances in carbon dioxide capture, fixation, and activation by using N-heterocyclic carbenes.ChemSusChem. 2014 Apr;7(4):962-98. doi: 10.1002/cssc.201301131. Epub 2014 Mar 18. ChemSusChem. 2014. PMID: 24644039 Review.
-
Mononuclear Cu Complexes Based on Nitrogen Heterocyclic Carbene: A Comprehensive Review.Top Curr Chem (Cham). 2020 May 4;378(3):39. doi: 10.1007/s41061-020-00304-8. Top Curr Chem (Cham). 2020. Retraction in: Top Curr Chem (Cham). 2020 Sep 2;378(4-5):45. doi: 10.1007/s41061-020-00308-4. PMID: 32367181 Retracted. Review.
Cited by
-
N‑Heterocyclic Carbene-Based Group 4 Catalysts for the Terpolymerization of Cyclohexene Oxide and Cyclic Anhydrides with CO2.ACS Org Inorg Au. 2025 May 13;5(3):171-180. doi: 10.1021/acsorginorgau.5c00002. eCollection 2025 Jun 4. ACS Org Inorg Au. 2025. PMID: 40487033 Free PMC article.
-
Mesoionic Carbenes in Low- to High-Valent Vanadium Chemistry.Inorg Chem. 2021 Oct 18;60(20):15421-15434. doi: 10.1021/acs.inorgchem.1c02087. Epub 2021 Sep 30. Inorg Chem. 2021. PMID: 34590834 Free PMC article.
-
Carbon Ligands: From Fundamental Aspects to Applications.Molecules. 2021 Apr 8;26(8):2132. doi: 10.3390/molecules26082132. Molecules. 2021. PMID: 33917652 Free PMC article.
-
Synthesis, Structural and Physicochemical Characterization of a Titanium(IV) Compound with the Hydroxamate Ligand N,2-Dihydroxybenzamide.Molecules. 2021 Sep 15;26(18):5588. doi: 10.3390/molecules26185588. Molecules. 2021. PMID: 34577059 Free PMC article.
-
Ring-Opening Polymerization of Cyclohexene Oxide and Cycloaddition with CO2 Catalyzed by Amine Triphenolate Iron(III) Complexes.Molecules. 2024 May 4;29(9):2139. doi: 10.3390/molecules29092139. Molecules. 2024. PMID: 38731630 Free PMC article.
References
-
- Díez-González S., Nolan S.P. Stereoelectronic Parameters Associated with N-heterocyclic Carbene (NHC) Ligands: A Quest for Understanding. Coord. Chem. Rev. 2007;251:874–883. doi: 10.1016/j.ccr.2006.10.004. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

