The Effects of Asbestos Fibers on Human T Cells
- PMID: 32977478
- PMCID: PMC7584019
- DOI: 10.3390/ijms21196987
The Effects of Asbestos Fibers on Human T Cells
Abstract
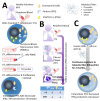
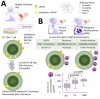
Asbestos exposure causes malignant tumors such as lung cancer and malignant mesothelioma. The effects of asbestos fibers on immunocompetent cells, however, have not been well studied. Asbestos physically comprises a fibrous substance, which differs from silica particles which are a particulate substance, although chemically it is a mineral silicate. Since silicosis patients previously exposed to silica particles often suffer from lung and autoimmune diseases, it is clear that silica exposure impairs immune tolerance. Similarly, asbestos may alter the immune system in asbestos-exposed individuals. Given that malignant tumors can result following exposure to asbestos, the attenuation of anti-tumor immunity in cases of asbestos exposure is an important area of investigation. We observed the effect of asbestos fibers on T lymphocytes, such as CD8+ cytotoxic T lymphocytes (CTLs), CD4+ helper T (Th), and regulatory T (Treg) cells, and showed that anti-tumor immunity was attenuated, as demonstrated in a system that stimulates fresh cells isolated from peripheral blood in vitro and a system that is continuously exposed to a cell line. In this manuscript, we introduce the experiments and results of studies on CTLs, as well as Th and Treg cells, and discuss how future changes in immunocompetent cells induced by asbestos fibers can be clinically linked.
Keywords: CD4+ T cell; T cell; asbestos; cytotoxic T lymphocyte; malignant mesothelioma; pleural plaque; regulatory T cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Induction of IL-17 production from human peripheral blood CD4+ cells by asbestos exposure.Int J Oncol. 2017 Jun;50(6):2024-2032. doi: 10.3892/ijo.2017.3991. Epub 2017 May 9. Int J Oncol. 2017. PMID: 28498408
-
Accelerated cell cycle progression of human regulatory T cell-like cell line caused by continuous exposure to asbestos fibers.Int J Oncol. 2017 Jan;50(1):66-74. doi: 10.3892/ijo.2016.3776. Epub 2016 Nov 22. Int J Oncol. 2017. PMID: 27878235 Free PMC article.
-
Effect of asbestos exposure on differentiation and function of cytotoxic T lymphocytes.Environ Health Prev Med. 2020 Oct 8;25(1):59. doi: 10.1186/s12199-020-00900-6. Environ Health Prev Med. 2020. PMID: 33032525 Free PMC article. Review.
-
Search for biomarkers of asbestos exposure and asbestos-induced cancers in investigations of the immunological effects of asbestos.Environ Health Prev Med. 2017 Jun 9;22(1):53. doi: 10.1186/s12199-017-0661-4. Environ Health Prev Med. 2017. PMID: 29165150 Free PMC article. Review.
-
Inflammatory Alteration of Human T Cells Exposed Continuously to Asbestos.Int J Mol Sci. 2018 Feb 8;19(2):504. doi: 10.3390/ijms19020504. Int J Mol Sci. 2018. PMID: 29419731 Free PMC article. Review.
Cited by
-
Amphibole asbestos as an environmental trigger for systemic autoimmune diseases.Autoimmun Rev. 2024 Jul-Aug;23(7-8):103603. doi: 10.1016/j.autrev.2024.103603. Epub 2024 Aug 20. Autoimmun Rev. 2024. PMID: 39154740 Review.
-
Ingredients such as trehalose and hesperidin taken as supplements or foods reverse alterations in human T cells, reducing asbestos exposure-induced antitumor immunity.Int J Oncol. 2021 Apr;58(4):2. doi: 10.3892/ijo.2021.5182. Epub 2021 Feb 2. Int J Oncol. 2021. PMID: 33655329 Free PMC article.
-
Recent progress and perspectives on the mechanisms underlying Asbestos toxicity.Genes Environ. 2021 Oct 12;43(1):46. doi: 10.1186/s41021-021-00215-0. Genes Environ. 2021. PMID: 34641979 Free PMC article. Review.
-
Asbestos ban policies and mesothelioma mortality in Greece.BMC Public Health. 2024 Apr 26;24(1):1177. doi: 10.1186/s12889-024-18030-x. BMC Public Health. 2024. PMID: 38671450 Free PMC article.
-
Interpreting Immunoregulation in Lung Fibrosis: A New Branch of the Immune Model.Front Immunol. 2021 Aug 20;12:690375. doi: 10.3389/fimmu.2021.690375. eCollection 2021. Front Immunol. 2021. PMID: 34489937 Free PMC article. Review.
References
-
- Lemen R.A. Introduction: History of the use of asbestos. Med. Lav. 1997;88:288–292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

