Diabetic Retinopathy: The Role of Mitochondria in the Neural Retina and Microvascular Disease
- PMID: 32977483
- PMCID: PMC7598160
- DOI: 10.3390/antiox9100905
Diabetic Retinopathy: The Role of Mitochondria in the Neural Retina and Microvascular Disease
Abstract
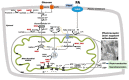
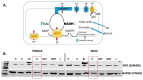
Diabetic retinopathy (DR), a common chronic complication of diabetes mellitus and the leading cause of vision loss in the working-age population, is clinically defined as a microvascular disease that involves damage of the retinal capillaries with secondary visual impairment. While its clinical diagnosis is based on vascular pathology, DR is associated with early abnormalities in the electroretinogram, indicating alterations of the neural retina and impaired visual signaling. The pathogenesis of DR is complex and likely involves the simultaneous dysregulation of multiple metabolic and signaling pathways through the retinal neurovascular unit. There is evidence that microvascular disease in DR is caused in part by altered energetic metabolism in the neural retina and specifically from signals originating in the photoreceptors. In this review, we discuss the main pathogenic mechanisms that link alterations in neural retina bioenergetics with vascular regression in DR. We focus specifically on the recent developments related to alterations in mitochondrial metabolism including energetic substrate selection, mitochondrial function, oxidation-reduction (redox) imbalance, and oxidative stress, and critically discuss the mechanisms of these changes and their consequences on retinal function. We also acknowledge implications for emerging therapeutic approaches and future research directions to find novel mitochondria-targeted therapeutic strategies to correct bioenergetics in diabetes. We conclude that retinal bioenergetics is affected in the early stages of diabetes with consequences beyond changes in ATP content, and that maintaining mitochondrial integrity may alleviate retinal disease.
Keywords: diabetic retinopathy; mitochondria; oxidative stress; photoreceptor; redox.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
-
- Royle P., Mistry H., Auguste P., Shyangdan D., Freeman K., Lois N., Waugh N. Pan-retinal photocoagulation and other forms of laser treatment and drug therapies for non-proliferative diabetic retinopathy: Systematic review and economic evaluation. Health Technol. Assess. 2015;19:1–247. doi: 10.3310/hta19510. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

