Syndecan-1 Promotes Hepatocyte-Like Differentiation of Hepatoma Cells Targeting Ets-1 and AP-1
- PMID: 32977498
- PMCID: PMC7598270
- DOI: 10.3390/biom10101356
Syndecan-1 Promotes Hepatocyte-Like Differentiation of Hepatoma Cells Targeting Ets-1 and AP-1
Abstract
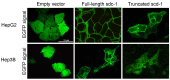
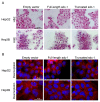
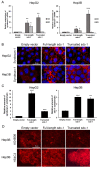
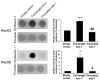
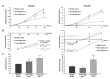
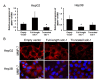
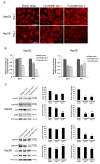
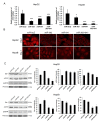
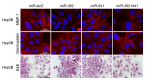
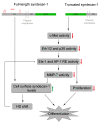
Syndecan-1 is a transmembrane heparan sulfate proteoglycan which is indispensable in the structural and functional integrity of epithelia. Normal hepatocytes display strong cell surface expression of syndecan-1; however, upon malignant transformation, they may lose it from their cell surfaces. In this study, we demonstrate that re-expression of full-length or ectodomain-deleted syndecan-1 in hepatocellular carcinoma cells downregulates phosphorylation of ERK1/2 and p38, with the truncated form exerting an even stronger effect than the full-length protein. Furthermore, overexpression of syndecan-1 in hepatoma cells is associated with a shift of heparan sulfate structure toward a highly sulfated type specific for normal liver. As a result, cell proliferation and proteolytic shedding of syndecan-1 from the cell surface are restrained, which facilitates redifferentiation of hepatoma cells to a more hepatocyte-like phenotype. Our results highlight the importance of syndecan-1 in the formation and maintenance of differentiated epithelial characteristics in hepatocytes partly via the HGF/ERK/Ets-1 signal transduction pathway. Downregulation of Ets-1 expression alone, however, was not sufficient to replicate the phenotype of syndecan-1 overexpressing cells, indicating the need for additional molecular mechanisms. Accordingly, a reporter gene assay revealed the inhibition of Ets-1 as well as AP-1 transcription factor-induced promoter activation, presumably an effect of the heparan sulfate switch.
Keywords: AP-1; Ets-1; MMP-7; differentiation; epithelium; heparan sulfate; liver cancer; shedding; syndecan-1.
Conflict of interest statement
The author (L.S.) is founder of Szilak Laboratories Bioinformatics and Molecule-Design Ltd. This founder provided the syndecan-1 constructs taking part in the design of the study. The author (P.T.) is employed by Solvo Biotechnology. The funder (Solvo Biotechnology) provided support in the form of author’s salaries for the author (P.T.), but did not have any additional role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The remaining authors declare no conflict of interest.
Figures

Similar articles
-
Heparanase-1-induced shedding of heparan sulfate from syndecan-1 in hepatocarcinoma cell facilitates lymphatic endothelial cell proliferation via VEGF-C/ERK pathway.Biochem Biophys Res Commun. 2017 Apr 1;485(2):432-439. doi: 10.1016/j.bbrc.2017.02.060. Epub 2017 Feb 13. Biochem Biophys Res Commun. 2017. PMID: 28209511
-
Id1 is down-regulated by hepatocyte growth factor via ERK-dependent and ERK-independent signaling pathways, leading to increased expression of p16INK4a in hepatoma cells.Mol Cancer Res. 2009 Jul;7(7):1179-88. doi: 10.1158/1541-7786.MCR-08-0289. Epub 2009 Jun 30. Mol Cancer Res. 2009. PMID: 19567783
-
Cell surface proteoglycan syndecan-1 mediates hepatocyte growth factor binding and promotes Met signaling in multiple myeloma.Blood. 2002 Feb 15;99(4):1405-10. doi: 10.1182/blood.v99.4.1405. Blood. 2002. PMID: 11830493
-
[Proteoglycans in the liver].Magy Onkol. 2004;48(3):207-13. Epub 2004 Nov 1. Magy Onkol. 2004. PMID: 15520870 Review. Hungarian.
-
The heparanase/syndecan-1 axis in cancer: mechanisms and therapies.FEBS J. 2013 May;280(10):2294-306. doi: 10.1111/febs.12168. Epub 2013 Mar 4. FEBS J. 2013. PMID: 23374281 Free PMC article. Review.
Cited by
-
Proteoglycans: Systems-Level Insight into Their Expression in Healthy and Diseased Placentas.Int J Mol Sci. 2022 May 21;23(10):5798. doi: 10.3390/ijms23105798. Int J Mol Sci. 2022. PMID: 35628608 Free PMC article. Review.
-
Development and Validation of a Prognostic Classifier Based on Lipid Metabolism-Related Genes for Breast Cancer.J Inflamm Res. 2022 Jun 14;15:3477-3499. doi: 10.2147/JIR.S357144. eCollection 2022. J Inflamm Res. 2022. PMID: 35726216 Free PMC article.
-
Overexpression of Human Syndecan-1 Protects against the Diethylnitrosamine-Induced Hepatocarcinogenesis in Mice.Cancers (Basel). 2021 Mar 27;13(7):1548. doi: 10.3390/cancers13071548. Cancers (Basel). 2021. PMID: 33801718 Free PMC article.
-
Diamond Nanofilm Normalizes Proliferation and Metabolism in Liver Cancer Cells.Nanotechnol Sci Appl. 2021 Aug 28;14:115-137. doi: 10.2147/NSA.S322766. eCollection 2021. Nanotechnol Sci Appl. 2021. PMID: 34511890 Free PMC article.
References
-
- Theise N.D., Curado M.P., Franceschi S., Hytiroglou P., Kudo M., Park Y.N., Sakamoto M., Torbenson M., Wee A. Tumours of the liver and intrahepatic bile ducts: Hepatocellular carcinoma. In: Bosman F.T., Carneiro F., Hruban R.H., Theise N.D., editors. World Health Organization Classification of Tumours of the Digestive System. 4th ed. Volume 3. IARC Press; Lyon, France: 2010. pp. 205–216.
-
- Rapraeger A., Jalkanen M., Endo E., Koda J., Bernfield M. The cell surface proteoglycan from mouse mammary epithelial cells bears chondroitin sulfate and heparan sulfate glycosaminoglycans. J. Biol. Chem. 1985;260:11046–11052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

