Cytotoxic and Senolytic Effects of Methadone in Combination with Temozolomide in Glioblastoma Cells
- PMID: 32977591
- PMCID: PMC7582495
- DOI: 10.3390/ijms21197006
Cytotoxic and Senolytic Effects of Methadone in Combination with Temozolomide in Glioblastoma Cells
Abstract
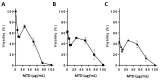
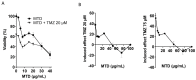
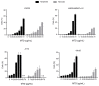
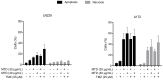
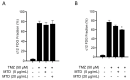
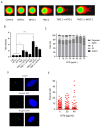
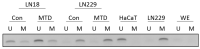
Methadone is an analgesic drug used for pain treatment and heroin substitution. Recently, methadone has been proposed to be useful also for cancer therapy, including glioblastoma multiforme (GBM), the most severe form of brain cancer, because experiments on cultured glioma cells treated with doxorubicin showed promising results. Doxorubicin, however, is not used first-line in GBM therapy. Therefore, we analyzed the cytotoxic effect of methadone alone and in combination with temozolomide, a DNA-alkylating drug that is first-line used in GBM treatment, utilizing GBM-derived cell lines and a human fibroblast cell line. We show that methadone is cytotoxic on its own, inducing apoptosis and necrosis, which was observed at a concentration above 20 µg/mL. Methadone was similar toxic in isogenic MGMT expressing and non-expressing cells, and in LN229 glioblastoma and VH10T human fibroblasts. The apoptosis-inducing activity of methadone is not bound on the opioid receptor (OR), since naloxone, a competitive inhibitor of OR, did not attenuate methadone-induced apoptosis/necrosis. Administrating methadone and temozolomide together, temozolomide had no impact on methadone-induced apoptosis (which occurred 3 days after treatment), while temozolomide-induced apoptosis (which occurred 5 days after treatment) was unaffected at low (non-toxic) methadone concentration (5 µg/mL), and at high (toxic) methadone concentration (20 µg/mL) the cytotoxic effects of methadone and temozolomide were additive. Methadone is not genotoxic, as revealed by comet and γH2AX assay, and did not ameliorate the genotoxic effect of temozolomide. Further, methadone did not induce cellular senescence and had no effect on temozolomide-induced senescence. Although methadone was toxic on senescent cells, it cannot be considered a senolytic drug since cytotoxicity was not specific for senescent cells. Finally, we show that methadone had no impact on the MGMT promoter methylation. Overall, the data show that methadone on glioblastoma cells in vitro is cytotoxic and induces apoptosis/necrosis at doses that are above the level that can be achieved in vivo. It is not genotoxic, and does not ameliorate the cell killing or the senescence-inducing effect of temozolomide (no synergistic effect), indicating it has no impact on temozolomide-induced signaling pathways. The data do not support the notion that concomitant methadone treatment supports temozolomide-based chemotherapy.
Keywords: apoptosis; cancer therapy; drug resistance; glioblastoma; methadone; senescence; temozolomide.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The opinions mentioned throughout the following article are personal views of the authors and do not reflect an official position of the Federal Institute for Drugs and Medical Devices or an EMA-committee or working party, respectively.
Figures




References
-
- Friesen C., Hormann I., Roscher M., Fichtner I., Alt A., Hilger R., Debatin K.-M., Miltner E. Opioid receptor activation triggering downregulation of cAMP improves effectiveness of anti-cancer drugs in treatment of glioblastoma. Cell Cycle. 2014;13:1560–1570. doi: 10.4161/cc.28493. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

