Identification of Modulators of HIV-1 Proviral Transcription from a Library of FDA-Approved Pharmaceuticals
- PMID: 32977702
- PMCID: PMC7598649
- DOI: 10.3390/v12101067
Identification of Modulators of HIV-1 Proviral Transcription from a Library of FDA-Approved Pharmaceuticals
Abstract
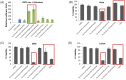
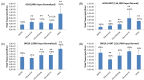
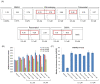
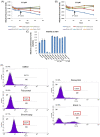
Human immunodeficiency virus 1 (HIV-1) is the most prevalent human retrovirus. Recent data show that 34 million people are living with HIV-1 worldwide. HIV-1 infections can lead to AIDS which still causes nearly 20,000 deaths annually in the USA alone. As this retrovirus leads to high morbidity and mortality conditions, more effective therapeutic regimens must be developed to treat these viral infections. A key target for intervention for which there are no current FDA-approved modulators is at the point of proviral transcription. One successful method for identifying novel therapeutics for treating infectious diseases is the repurposing of pharmaceuticals that are approved by the FDA for alternate indications. Major benefits of using FDA-approved drugs include the fact that the compounds have well established toxicity profiles, approved manufacturing processes, and immediate commercial availability to the patients. Here, we demonstrate that pharmaceuticals previously approved for other indications can be utilized to either activate or inhibit HIV-1 proviral transcription. Specifically, we found febuxostat, eltrombopag, and resveratrol to be activators of HIV-1 transcription, while mycophenolate was our lead inhibitor of HIV-1 transcription. Additionally, we observed that the infected cells of lymphoid and myeloid lineage responded differently to our lead transcriptional modulators. Finally, we demonstrated that the use of a multi-dose regimen allowed for enhanced activation with our transcriptional activators.
Keywords: HIV; activator; eltrombopag; febuxostat; inhibitor; latency; mycophenolate; resveratrol; transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Joint United Nations Programme on HIV/AIDS . United Nations Global Report: UNAIDS Report on the Global AIDS Epidemic: 2012. UNAIDS; Geneva, Switzerland: 2012.
-
- Heaton R.K., Clifford D.B., Franklin D.R., Woods S.P., Ake C., Vaida F., Ellis R.J., Letendre S.L., Marcotte T.D., Atkinson J.H., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. - DOI - PMC - PubMed
-
- US. Federal Funding for HIV/AIDS . The President’s FY 2016 Budget Request. Health and Human Services; Washington, DC, USA: 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

