Taking the Hinge off: An Approach to Effector-Less Monoclonal Antibodies
- PMID: 32977708
- PMCID: PMC7709103
- DOI: 10.3390/antib9040050
Taking the Hinge off: An Approach to Effector-Less Monoclonal Antibodies
Abstract
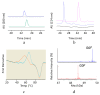
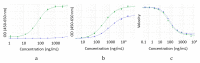
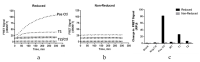
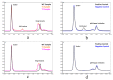
A variety of Fc domain engineering approaches for abrogating the effector functions of mAbs exists. To address some of the limitations of the current Fc domain silencing approaches, we are exploring a less commonly considered option which relies on the deletion of the hinge. Removal of the hinge domain in humanized IgG1 and IgG4 mAbs obliterates their ability to bind to activating human Fc gamma receptors I and IIIA, while leaving their ability to engage their target antigen intact. Deletion of the hinge also reduces binding to the Fc neonatal receptor, although Fc engineering allows partial recovery of affinity. Engineering of the CH3 domain, stabilizes hinge deleted IgG4s and prevents Fab arm exchange. The faster clearing properties together with the pacified Fc make modality of the hinge deleted mAb an appealing solution for therapeutic and diagnostic applications.
Keywords: Antibody-dependent cellular cytotoxicity (ADCC); Fab arm exchange; Fc neonatal receptor; FcRn; Fcγ receptors; FcγRI; FcγRIII; IgG1; IgG4; effector function; hinge; mAb; monoclonal antibody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations.Methods. 2014 Jan 1;65(1):114-26. doi: 10.1016/j.ymeth.2013.06.035. Epub 2013 Jul 17. Methods. 2014. PMID: 23872058
-
Effects of terminal galactose residues in mannose α1-6 arm of Fc-glycan on the effector functions of therapeutic monoclonal antibodies.MAbs. 2019 Jul;11(5):826-836. doi: 10.1080/19420862.2019.1608143. Epub 2019 May 8. MAbs. 2019. PMID: 30990348 Free PMC article.
-
Fc Engineering of Human IgG1 for Altered Binding to the Neonatal Fc Receptor Affects Fc Effector Functions.J Immunol. 2015 Jun 1;194(11):5497-508. doi: 10.4049/jimmunol.1401218. Epub 2015 Apr 22. J Immunol. 2015. PMID: 25904551 Free PMC article.
-
New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies.BioDrugs. 2011 Feb 1;25(1):13-25. doi: 10.2165/11539590-000000000-00000. BioDrugs. 2011. PMID: 21090841 Review.
-
Advances in Therapeutic Fc Engineering - Modulation of IgG-Associated Effector Functions and Serum Half-life.Front Immunol. 2016 Dec 12;7:580. doi: 10.3389/fimmu.2016.00580. eCollection 2016. Front Immunol. 2016. PMID: 28018347 Free PMC article. Review.
Cited by
-
Effects of glycans and hinge on dynamics in the IgG1 Fc.J Biomol Struct Dyn. 2024;42(22):12571-12579. doi: 10.1080/07391102.2023.2270749. Epub 2023 Oct 28. J Biomol Struct Dyn. 2024. PMID: 37897185 Free PMC article.
-
Hinge Truncation to Improve Aggregation Kinetics and Thermal Stability of an Antibody Fab Fragment.Mol Pharm. 2025 Sep 1;22(9):5389-5399. doi: 10.1021/acs.molpharmaceut.5c00358. Epub 2025 Aug 7. Mol Pharm. 2025. PMID: 40772589 Free PMC article.
-
Cryomicroscopy reveals the structural basis for a flexible hinge motion in the immunoglobulin M pentamer.Nat Commun. 2022 Oct 23;13(1):6314. doi: 10.1038/s41467-022-34090-2. Nat Commun. 2022. PMID: 36274064 Free PMC article.
-
Pathophysiological Effects of Autoantibodies in Autoimmune Encephalitides.Cells. 2023 Dec 20;13(1):15. doi: 10.3390/cells13010015. Cells. 2023. PMID: 38201219 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

