Implant contamination as a cause of surgical site infection in spinal surgery: are single-use implants a reasonable solution? - a systematic review
- PMID: 32977778
- PMCID: PMC7519515
- DOI: 10.1186/s12891-020-03653-z
Implant contamination as a cause of surgical site infection in spinal surgery: are single-use implants a reasonable solution? - a systematic review
Abstract
Background: In spine surgery, surgical site infection (SSI) is one of the main perioperative complications and is associated with a higher patient morbidity and longer patient hospitalization. Most factors associated with SSI are connected with asepsis during the surgical procedure and thus with contamination of implants and instruments used which can be caused by pre- and intraoperative factors. In this systematic review we evaluate the current literature on these causes and discuss possible solutions to avoid implant and instrument contamination.
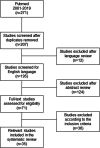
Methods: A systematic literature search of PubMed addressing implant, instrument and tray contamination in orthopaedic and spinal surgery from 2001 to 2019 was conducted following the PRISMA guidelines. All studies regarding implant and instrument contamination in orthopaedic surgery published in English language were included.
Results: Thirty-five studies were eligible for inclusion and were divided into pre- and intraoperative causes for implant and instrument contamination. Multiple studies showed that reprocessing of medical devices for surgery may be insufficient and lead to surgical site contamination. Regarding intraoperative causes, contamination of gloves and gowns as well as contamination via air are the most striking factors contributing to microbial contamination.
Conclusions: Our systematic literature review shows that multiple factors can lead to instrument or implant contamination. Intraoperative causes of contamination can be avoided by implementing behavior such as changing gloves right before handling an implant and reducing the instruments' intraoperative exposure to air. In avoidance of preoperative contamination, there still is a lack of convincing evidence for the use of single-use implants in orthopaedic surgery.
Keywords: Bacteria; Implant contamination; Single-use implants; Spinal surgery; Sterilization.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Implant Prophylaxis: The Next Best Practice Toward Asepsis in Spine Surgery.Global Spine J. 2018 Oct;8(7):761-765. doi: 10.1177/2192568218762380. Epub 2018 Apr 24. Global Spine J. 2018. PMID: 30443488 Free PMC article. Review.
-
Glove change to reduce the risk of surgical site infection or prosthetic joint infection in arthroplasty surgeries: a systematic review.ANZ J Surg. 2019 Sep;89(9):1009-1015. doi: 10.1111/ans.14936. Epub 2018 Nov 29. ANZ J Surg. 2019. PMID: 30497094
-
Updates on Evidence-Based Practices to Reduce Preoperative and Intraoperative Contamination of Implants in Spine Surgery: A Narrative Review.Spine Surg Relat Res. 2019 Jun 21;4(2):111-116. doi: 10.22603/ssrr.2019-0038. eCollection 2020. Spine Surg Relat Res. 2019. PMID: 32405555 Free PMC article. Review.
-
Surgical site infection after total en bloc spondylectomy: risk factors and the preventive new technology.Spine J. 2015 Jan 1;15(1):132-7. doi: 10.1016/j.spinee.2014.08.007. Epub 2014 Aug 15. Spine J. 2015. PMID: 25131266
-
Intraoperative Surgical Wound Contamination May Not Lead to Surgical-Site Infection in Patients Undergoing Clean Orthopaedic Procedures.J Lab Physicians. 2022 Feb 9;14(3):284-289. doi: 10.1055/s-0042-1742422. eCollection 2022 Sep. J Lab Physicians. 2022. PMID: 36119426 Free PMC article.
Cited by
-
In Vivo Prevention of Implant-Associated Infections Caused by Antibiotic-Resistant Bacteria through Biofunctionalization of Additively Manufactured Porous Titanium.J Funct Biomater. 2023 Oct 16;14(10):520. doi: 10.3390/jfb14100520. J Funct Biomater. 2023. PMID: 37888185 Free PMC article.
-
Postoperative Spinal Implant Infections (PSII)-A Systematic Review: What Do We Know So Far and What is Critical About It?Global Spine J. 2022 Jul;12(6):1231-1246. doi: 10.1177/21925682211024198. Epub 2021 Jun 21. Global Spine J. 2022. PMID: 34151619 Free PMC article.
-
Optimisation of perioperative procedural factors to reduce the risk of surgical site infection in patients undergoing surgery: a systematic review.Discov Health Syst. 2023;2(1):6. doi: 10.1007/s44250-023-00019-9. Epub 2023 Feb 13. Discov Health Syst. 2023. PMID: 37520513 Free PMC article. Review.
-
Artificial Intelligence Machine Learning Algorithms Versus Standard Linear Demographic Analysis in Predicting Implant Size of Anatomic and Reverse Total Shoulder Arthroplasty.J Am Acad Orthop Surg Glob Res Rev. 2024 Aug 1;8(8):e24.00182. doi: 10.5435/JAAOSGlobal-D-24-00182. eCollection 2024 Aug 1. J Am Acad Orthop Surg Glob Res Rev. 2024. PMID: 39106479 Free PMC article.
-
Is Iatrogenic Implant Contamination Preventable Using a 16-Step No-Touch Protocol?Eplasty. 2022 Aug 24;22:e38. eCollection 2022. Eplasty. 2022. PMID: 36160667 Free PMC article.
References
-
- McClelland S, 3rd, Takemoto RC, Lonner BS, Andres TM, Park JJ, Ricart-Hoffiz PA, Bendo JA, Goldstein JA, Spivak JM, Errico T. Analysis of postoperative thoracolumbar spine infections in a prospective randomized controlled trial using the centers for disease control surgical site infection criteria. Int J Spine Surg. 2016;10:14. doi: 10.14444/3014. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources