Antimicrobial resistance in Clostridioides (Clostridium) difficile derived from humans: a systematic review and meta-analysis
- PMID: 32977835
- PMCID: PMC7517813
- DOI: 10.1186/s13756-020-00815-5
Antimicrobial resistance in Clostridioides (Clostridium) difficile derived from humans: a systematic review and meta-analysis
Abstract
Background: Clostridioides (Clostridium) difficile is an important pathogen of healthcare- associated diarrhea, however, an increase in the occurrence of C. difficile infection (CDI) outside hospital settings has been reported. The accumulation of antimicrobial resistance in C. difficile can increase the risk of CDI development and/or its spread. The limited number of antimicrobials for the treatment of CDI is matter of some concern.
Objectives: In order to summarize the data on antimicrobial resistance to C. difficile derived from humans, a systematic review and meta-analysis were performed.
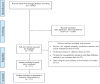
Methods: We searched five bibliographic databases: (MEDLINE [PubMed], Scopus, Embase, Cochrane Library and Web of Science) for studies that focused on antimicrobial susceptibility testing in C. difficile and were published between 1992 and 2019. The weighted pooled resistance (WPR) for each antimicrobial agent was calculated using a random- effects model.
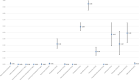
Results: A total of 111 studies were included. The WPR for metronidazole and vancomycin was 1.0% (95% CI 0-3%) and 1% (95% CI 0-2%) for the breakpoint > 2 mg/L and 0% (95% CI 0%) for breakpoint ≥32 μg/ml. Rifampin and tigecycline had a WPRs of 37.0% (95% CI 18-58%) and 1% (95% CI 0-3%), respectively. The WPRs for the other antimicrobials were as follows: ciprofloxacin 95% (95% CI 85-100%), moxifloxacin 32% (95% CI 25-40%), clindamycin 59% (95% CI 53-65%), amoxicillin/clavulanate 0% (0-0%), piperacillin/tazobactam 0% (0-0%) and ceftriaxone 47% (95% CI 29-65%). Tetracycline had a WPR 20% (95% CI 14-27%) and meropenem showed 0% (95% CI 0-1%); resistance to fidaxomicin was reported in one isolate (0.08%).
Conclusion: Resistance to metronidazole, vancomycin, fidaxomicin, meropenem and piperacillin/tazobactam is reported rarely. From the alternative CDI drug treatments, tigecycline had a lower resistance rate than rifampin. The high-risk antimicrobials for CDI development showed a high level of resistance, the highest was seen in the second generation of fluoroquinolones and clindamycin; amoxicillin/clavulanate showed almost no resistance. Tetracycline resistance was present in one fifth of human clinical C. difficile isolates.
Keywords: Antimicrobial resistance; Clostridioides difficile; Meta-analysis; Metronidazole; Vancomycin.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Antimicrobial resistance progression in the United Kingdom: A temporal comparison of Clostridioides difficile antimicrobial susceptibilities.Anaerobe. 2021 Aug;70:102385. doi: 10.1016/j.anaerobe.2021.102385. Epub 2021 May 25. Anaerobe. 2021. PMID: 34048922
-
Prevalence and antimicrobial resistance pattern of Clostridium difficile among hospitalized diarrheal patients: A systematic review and meta-analysis.PLoS One. 2022 Jan 13;17(1):e0262597. doi: 10.1371/journal.pone.0262597. eCollection 2022. PLoS One. 2022. PMID: 35025959 Free PMC article.
-
Antimicrobial resistance surveillance of Clostridioides difficile in Australia, 2015-18.J Antimicrob Chemother. 2021 Jun 18;76(7):1815-1821. doi: 10.1093/jac/dkab099. J Antimicrob Chemother. 2021. PMID: 33895826
-
Spore-Forming Clostridium (Clostridioides) difficile in Wastewater Treatment Plants in Western Australia.Microbiol Spectr. 2023 Feb 14;11(1):e0358222. doi: 10.1128/spectrum.03582-22. Epub 2022 Dec 8. Microbiol Spectr. 2023. PMID: 36475924 Free PMC article.
-
The incidence and drug resistance of Clostridium difficile infection in Mainland China: a systematic review and meta-analysis.Sci Rep. 2016 Nov 29;6:37865. doi: 10.1038/srep37865. Sci Rep. 2016. PMID: 27897206 Free PMC article.
Cited by
-
Antibiotic Resistances of Clostridioides difficile.Adv Exp Med Biol. 2024;1435:169-198. doi: 10.1007/978-3-031-42108-2_9. Adv Exp Med Biol. 2024. PMID: 38175476
-
Molecular Epidemiology and Antimicrobial Resistance of Clostridioides difficile in Hospitalized Patients From Mexico.Front Microbiol. 2022 Mar 10;12:787451. doi: 10.3389/fmicb.2021.787451. eCollection 2021. Front Microbiol. 2022. PMID: 35360652 Free PMC article.
-
Role of previous systemic antibiotic therapy on the probability of recurrence after an initial episode of Clostridioides difficile infection treated with vancomycin.JAC Antimicrob Resist. 2023 Mar 23;5(2):dlad033. doi: 10.1093/jacamr/dlad033. eCollection 2023 Apr. JAC Antimicrob Resist. 2023. PMID: 36968953 Free PMC article.
-
Clostridioides difficile evolution in a tertiary German hospital through a retrospective genomic characterization.Infection. 2025 Jun 8. doi: 10.1007/s15010-025-02576-y. Online ahead of print. Infection. 2025. PMID: 40483626
-
Molecular epidemiology and clinical characteristics of Clostridioides difficile infection in patients with inflammatory bowel disease from a teaching hospital.J Clin Lab Anal. 2022 Dec;36(12):e24773. doi: 10.1002/jcla.24773. Epub 2022 Nov 17. J Clin Lab Anal. 2022. PMID: 36397282 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical