Synergistic Action of a Lytic Polysaccharide Monooxygenase and a Cellobiohydrolase from Penicillium funiculosum in Cellulose Saccharification under High-Level Substrate Loading
- PMID: 32978122
- PMCID: PMC7657632
- DOI: 10.1128/AEM.01769-20
Synergistic Action of a Lytic Polysaccharide Monooxygenase and a Cellobiohydrolase from Penicillium funiculosum in Cellulose Saccharification under High-Level Substrate Loading
Abstract
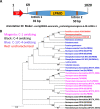
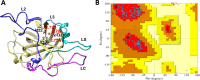
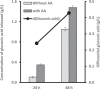
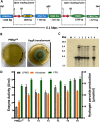
Lytic polysaccharide monooxygenases (LPMOs) are crucial industrial enzymes required in the biorefinery industry as well as in the natural carbon cycle. These enzymes, known to catalyze the oxidative cleavage of glycosidic bonds, are produced by numerous bacterial and fungal species to assist in the degradation of cellulosic biomass. In this study, we annotated and performed structural analysis of an uncharacterized LPMO from Penicillium funiculosum (PfLPMO9) based on computational methods in an attempt to understand the behavior of this enzyme in biomass degradation. PfLPMO9 exhibited 75% and 36% sequence identities with LPMOs from Thermoascus aurantiacus (TaLPMO9A) and Lentinus similis (LsLPMO9A), respectively. Furthermore, multiple fungal genetic manipulation tools were employed to simultaneously overexpress LPMO and cellobiohydrolase I (CBH1) in a catabolite-derepressed strain of Penicillium funiculosum, PfMig188 (an engineered variant of P. funiculosum), to improve its saccharification performance toward acid-pretreated wheat straw (PWS) at 20% substrate loading. The resulting transformants showed improved LPMO and CBH1 expression at both the transcriptional and translational levels, with ∼200% and ∼66% increases in ascorbate-induced LPMO and Avicelase activities, respectively. While the secretome of PfMig88 overexpressing LPMO or CBH1 increased the saccharification of PWS by 6% or 13%, respectively, over the secretome of PfMig188 at the same protein concentration, the simultaneous overexpression of these two genes led to a 20% increase in saccharification efficiency over that observed with PfMig188, which accounted for 82% saccharification of PWS under 20% substrate loading.IMPORTANCE The enzymatic hydrolysis of cellulosic biomass by cellulases continues to be a significant bottleneck in the development of second-generation biobased industries. While increasing efforts are being made to obtain indigenous cellulases for biomass hydrolysis, the high production cost of this enzyme remains a crucial challenge affecting its wide availability for the efficient utilization of cellulosic materials. This is because it is challenging to obtain an enzymatic cocktail with balanced activity from a single host. This report describes the annotation and structural analysis of an uncharacterized lytic polysaccharide monooxygenase (LPMO) gene in Penicillium funiculosum and its impact on biomass deconstruction upon overexpression in a catabolite-derepressed strain of P. funiculosum Cellobiohydrolase I (CBH1), which is the most important enzyme produced by many cellulolytic fungi for the saccharification of crystalline cellulose, was further overexpressed simultaneously with LPMO. The resulting secretome was analyzed for enhanced LPMO and exocellulase activities and the corresponding improvement in saccharification performance (by ∼20%) under high-level substrate loading using a minimal amount of protein.
Keywords: CBH1; LPMO; Penicillium funiculosum; PfMig188; cellobiohydrolase I; cellulase; fungi; fungus; lytic polysaccharide monooxygenase; saccharification.
Copyright © 2020 American Society for Microbiology.
Figures



References
-
- Chandra M, Kalra A, Sharma PK, Kumar H, Sangwan RS. 2010. Optimization of cellulases production by Trichoderma citrinoviride on marc of Artemisia annua and its application for bioconversion process. Biomass Bioenergy 34:805–811. doi:10.1016/j.biombioe.2010.01.024. - DOI
-
- Srivastava N, Srivastava M, Mishra PK, Gupta VK, Molina G, Rodriguez-Couto S, Manikanta A, Ramteke PW. 2018. Applications of fungal cellulases in biofuel production: advances and limitations. Renew Sustain Energy Rev 82:2379–2386. doi:10.1016/j.rser.2017.08.074. - DOI
-
- El-Hadi AA, El-Nour SA, Hammad A, Kamel Z, Anwar M. 2014. Optimization of cultural and nutritional conditions for carboxymethylcellulase production by Aspergillus hortai. J Radiat Res Appl Sci 7:23–28. doi:10.1016/j.jrras.2013.11.003. - DOI
-
- Bozell JJ, Petersen GR. 2010. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “top 10” revisited. Green Chem 12:539–554. doi:10.1039/b922014c. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

