Biofilm Sampling for Detection of Cryptosporidium Oocysts in a Southeastern Pennsylvania Watershed
- PMID: 32978132
- PMCID: PMC7657627
- DOI: 10.1128/AEM.01399-20
Biofilm Sampling for Detection of Cryptosporidium Oocysts in a Southeastern Pennsylvania Watershed
Abstract
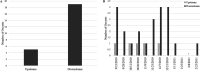
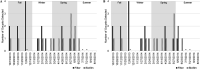
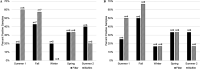
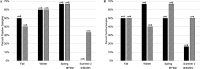
This study investigated the use of biofilms to monitor Cryptosporidium in water. Benthic rock and submersible slide biofilms were sampled upstream and downstream of point sources in a suburban watershed in southeastern Pennsylvania. More oocysts were detected in biofilms scraped from rocks downstream than upstream of a wastewater treatment plant (WWTP) (19 versus 5, respectively; n = 1). Although not statistically significant, Cryptosporidium oocysts were detected more frequently, and in greater numbers, in biofilms grown on slides downstream than upstream of this same WWTP (83.3% positive samples [n = 12] versus 45.5% positive samples [n = 11], respectively; P = 0.0567). Similarly, Cryptosporidium oocysts were detected more frequently, and in greater numbers, in rock biofilms collected downstream than upstream of a stormwater outfall impacted by defective sewer laterals (50% positive samples downstream and 17% positive samples upstream; n = 6; P = 0.2207). While oocyst detection data obtained by slide biofilms versus filters did not necessarily agree on a given day, there was no seasonal difference in the frequency of oocyst detection (P > 0.05) or numbers of oocysts detected (P > 0.05) whether the water was monitored by filtration or slide biofilm sampling. Within any given season, there was no difference in the frequency of oocyst detection (P > 0.05) or the numbers of oocysts detected (P > 0.05) whether the water was monitored by filtration or slide biofilm sampling. These data show that oocyst detection in biofilms is comparable to oocyst detection in filtered water samples. Biofilm sampling offers significant cost savings compared to the filtration-based EPA Method 1623.1 and could be used to identify watershed locations at potential risk for increased oocyst loads.IMPORTANCE Monitoring Cryptosporidium occurrence in watersheds that provide drinking water is necessary to determine where limited resources should most effectively be directed to protect consumers from waterborne exposure to pathogenic oocysts. Biofilms are a useful tool to monitor complex watersheds and identify point sources of Cryptosporidium oocyst contamination that need to be managed to protect public health. Compared to EPA Method 1623.1, the cost benefit of using biofilms to monitor for Cryptosporidium contamination will enable utilities to sample water supplies more frequently, and at more locations, than is currently possible given limited operating budgets. Biofilm sampling could be used to identify high-risk regions within a large, complex watershed and the associated water treatment plants at potential risk for increased oocyst loads in the water supply; this information could then be used to select the locations within the watershed where the more expensive EPA Method 1623.1 is warranted.
Keywords: Cryptosporidium; biofilms; detection; filtration; monitoring; sampling; water.
Copyright © 2020 American Society for Microbiology.
Figures



Similar articles
-
Seasonal retention and release of Cryptosporidium parvum oocysts by environmental biofilms in the laboratory.Appl Environ Microbiol. 2010 Feb;76(4):1021-7. doi: 10.1128/AEM.01804-09. Epub 2009 Dec 18. Appl Environ Microbiol. 2010. PMID: 20023100 Free PMC article.
-
Detection of Giardia and Cryptosporidium cysts/oocysts in watersheds and drinking water sources in Brazil urban areas.J Water Health. 2010 Jun;8(2):399-404. doi: 10.2166/wh.2009.172. Epub 2009 Nov 9. J Water Health. 2010. PMID: 20154402
-
Cryptosporidium Attenuation across the Wastewater Treatment Train: Recycled Water Fit for Purpose.Appl Environ Microbiol. 2017 Feb 15;83(5):e03068-16. doi: 10.1128/AEM.03068-16. Print 2017 Mar 1. Appl Environ Microbiol. 2017. PMID: 28039137 Free PMC article.
-
Cryptosporidium parvum and Cyclospora cayetanensis: a review of laboratory methods for detection of these waterborne parasites.J Microbiol Methods. 2002 May;49(3):209-24. doi: 10.1016/s0167-7012(02)00007-6. J Microbiol Methods. 2002. PMID: 11869786 Review.
-
Biology, persistence and detection of Cryptosporidium parvum and Cryptosporidium hominis oocyst.Water Res. 2004 Feb;38(4):818-62. doi: 10.1016/j.watres.2003.10.012. Water Res. 2004. PMID: 14769405 Review.
References
-
- Edzwald JK, Kelley MB. 1998. Control of Cryptosporidium: from reservoirs to clarifiers to filters. Water Sci Technol 37(2):1–8. doi:10.2166/wst.1998.0089. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

