Novel Modifications of Nonribosomal Peptides from Brevibacillus laterosporus MG64 and Investigation of Their Mode of Action
- PMID: 32978140
- PMCID: PMC7688214
- DOI: 10.1128/AEM.01981-20
Novel Modifications of Nonribosomal Peptides from Brevibacillus laterosporus MG64 and Investigation of Their Mode of Action
Abstract
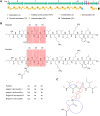
Nonribosomal peptides (NRPs) are a class of secondary metabolites usually produced by microorganisms. They are of paramount importance in different applications, including biocontrol and pharmacy. Brevibacillus spp. are a rich source of NRPs yet have received little attention. In this study, we characterize four novel bogorol variants (bogorols I to L, cationic linear lipopeptides) and four succilins (succilins I to L, containing a succinyl group that is attached to the Orn3/Lys3 in bogorols I to L) from the biocontrol strain Brevibacillus laterosporus MG64. Further investigation revealed that the bogorol family of peptides employs an adenylation pathway for lipoinitiation, different from the usual pattern, which is based on an external ligase and coenzyme A. Moreover, the formation of valinol was proven to be mediated by a terminal reductase domain and a reductase encoded by the bogI gene. Furthermore, succinylation, which is a novel type of modification in the family of bogorols, was discovered. Its occurrence requires a high concentration of the substrate (bogorols), but its responsible enzyme remains unknown. Bogorols display potent activity against both Gram-positive and Gram-negative bacteria. Investigation of their mode of action reveals that bogorols form pores in the cell membrane of both Gram-positive and Gram-negative bacteria. The combination of bogorols and relacidines, another class of NRPs produced by B. laterosporus MG64, displays a synergistic effect on different pathogens, suggesting the great potential of both peptides as well as their producer B. laterosporus MG64 for broad applications. Our study provides a further understanding of the bogorol family of peptides as well as their applications.IMPORTANCE NRPs form a class of secondary metabolites with biocontrol and pharmaceutical potential. This work describes the identification of novel bogorol variants and succinylated bogorols (namely, succilins) and further investigates their biosynthetic pathway and mode of action. Adenylation domain-mediated lipoinitiation of bogorols represents a novel pathway by which NRPs incorporate fatty acid tails. This pathway provides the possibility to engineer the lipid tail of NRPs without identifying a fatty acid coenzyme ligase, which is usually not present in the biosynthetic gene cluster. The terminal reductase domain (TD) and BogI-mediated valinol formation and their effect on the biological activity of bogorols are revealed. Succinylation, which is rarely reported in NRPs, was discovered in the bogorol family of peptides. We demonstrate that bogorols combat bacterial pathogens by forming pores in the cell membrane. We also report the synergistic effect of two natural products (relacidine B and bogorol K) produced by the same strain, which is relevant for competition for a niche.
Keywords: Brevibacillus laterosporus; biosynthesis; bogorol variants; lipoinitiation; mode of action; reduction; succilins; succinylation; synergy.
Copyright © 2020 American Society for Microbiology.
Figures



Similar articles
-
Characterization of two relacidines belonging to a novel class of circular lipopeptides that act against Gram-negative bacterial pathogens.Environ Microbiol. 2020 Dec;22(12):5125-5136. doi: 10.1111/1462-2920.15145. Epub 2020 Jul 20. Environ Microbiol. 2020. PMID: 32608161 Free PMC article.
-
Antibacterial and antitumor activity of Bogorol B-JX isolated from Brevibacillus laterosporus JX-5.World J Microbiol Biotechnol. 2017 Sep 18;33(10):177. doi: 10.1007/s11274-017-2337-z. World J Microbiol Biotechnol. 2017. PMID: 28921048
-
An accurate strategy for pointing the key biocatalytic sites of bre2691A protein for modification of the brevilaterin from Brevibacillus laterosporus.Microb Cell Fact. 2022 Sep 19;21(1):196. doi: 10.1186/s12934-022-01918-x. Microb Cell Fact. 2022. PMID: 36123650 Free PMC article.
-
Antimicrobial peptides produced by Brevibacillus spp.: structure, classification and bioactivity: a mini review.World J Microbiol Biotechnol. 2018 Mar 29;34(4):57. doi: 10.1007/s11274-018-2437-4. World J Microbiol Biotechnol. 2018. PMID: 29594558 Review.
-
Nonribosomal peptides and polyketides of Burkholderia: new compounds potentially implicated in biocontrol and pharmaceuticals.Environ Sci Pollut Res Int. 2018 Oct;25(30):29794-29807. doi: 10.1007/s11356-017-9166-3. Epub 2017 May 25. Environ Sci Pollut Res Int. 2018. PMID: 28547376 Review.
Cited by
-
Underexplored bacteria as reservoirs of novel antimicrobial lipopeptides.Front Chem. 2022 Oct 5;10:1025979. doi: 10.3389/fchem.2022.1025979. eCollection 2022. Front Chem. 2022. PMID: 36277345 Free PMC article. Review.
-
Exploring Structure-Activity Relationships and Modes of Action of Laterocidine.ACS Cent Sci. 2024 Aug 6;10(9):1703-1717. doi: 10.1021/acscentsci.4c00776. eCollection 2024 Sep 25. ACS Cent Sci. 2024. PMID: 39345814 Free PMC article.
-
Antibacterial Regularity Mining Beneath the Systematic Activity Database of Lipopeptides Brevilaterins: An Instructive Activity Handbook for Its Food Application.Foods. 2022 Sep 26;11(19):2991. doi: 10.3390/foods11192991. Foods. 2022. PMID: 36230066 Free PMC article.
-
BrevicidineB, a New Member of the Brevicidine Family, Displays an Extended Target Specificity.Front Microbiol. 2021 Jun 9;12:693117. doi: 10.3389/fmicb.2021.693117. eCollection 2021. Front Microbiol. 2021. PMID: 34177875 Free PMC article.
-
Nonribosomal Peptide Synthesis Definitely Working Out of the Rules.Microorganisms. 2022 Mar 7;10(3):577. doi: 10.3390/microorganisms10030577. Microorganisms. 2022. PMID: 35336152 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

