A machine learning approach to define antimalarial drug action from heterogeneous cell-based screens
- PMID: 32978158
- PMCID: PMC7518791
- DOI: 10.1126/sciadv.aba9338
A machine learning approach to define antimalarial drug action from heterogeneous cell-based screens
Abstract
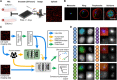
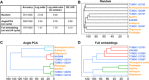
Drug resistance threatens the effective prevention and treatment of an ever-increasing range of human infections. This highlights an urgent need for new and improved drugs with novel mechanisms of action to avoid cross-resistance. Current cell-based drug screens are, however, restricted to binary live/dead readouts with no provision for mechanism of action prediction. Machine learning methods are increasingly being used to improve information extraction from imaging data. These methods, however, work poorly with heterogeneous cellular phenotypes and generally require time-consuming human-led training. We have developed a semi-supervised machine learning approach, combining human- and machine-labeled training data from mixed human malaria parasite cultures. Designed for high-throughput and high-resolution screening, our semi-supervised approach is robust to natural parasite morphological heterogeneity and correctly orders parasite developmental stages. Our approach also reproducibly detects and clusters drug-induced morphological outliers by mechanism of action, demonstrating the potential power of machine learning for accelerating cell-based drug discovery.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures


References
-
- Moffat J. G., Vincent F., Lee J. A., Eder J., Prunotto M., Opportunities and challenges in phenotypic drug discovery: An industry perspective. Nat. Rev. Drug Discov. 16, 531–543 (2017). - PubMed
-
- Antonova-Koch Y., Meister S., Abraham M., Luth M. R., Ottilie S., Lukens A. K., Sakata-Kato T., Vanaerschot M., Owen E., Jado J. C., Maher S. P., Calla J., Plouffe D., Zhong Y., Chen K., Chaumeau V., Conway A. J., McNamara C. W., Ibanez M., Gagaring K., Serrano F. N., Eribez K., Taggard C. M., Cheung A. L., Lincoln C., Ambachew B., Rouillier M., Siegel D., Nosten F., Kyle D. E., Gamo F.-J., Zhou Y., Llinás M., Fidock D. A., Wirth D. F., Burrows J., Campo B., Winzeler E. A., Open-source discovery of chemical leads for next-generation chemoprotective antimalarials. Science 362, eaat9446 (2018). - PMC - PubMed
-
- WHO, World Malaria Report (Geneva, 2019).
-
- Hamilton W. L., Amato R., van der Pluijm R. W., Jacob C. G., Quang H. H., Thuy-Nhien N. T., Hien T. T., Hongvanthong B., Chindavongsa K., Mayxay M., Huy R., Leang R., Huch C., Dysoley L., Amaratunga C., Suon S., Fairhurst R. M., Tripura R., Peto T. J., Sovann Y., Jittamala P., Hanboonkunupakarn B., Pukrittayakamee S., Chau N. H., Imwong M., Dhorda M., Vongpromek R., Chan X. H. S., Maude R. J., Pearson R. D., Nguyen T., Rockett K., Drury E., Gonçalves S., White N. J., Day N. P., Kwiatkowski D. P., Dondorp A. M., Miotto O., Evolution and expansion of multidrug-resistant malaria in southeast Asia: A genomic epidemiology study. Lancet Infect. Dis. 19, 943–951 (2019). - PMC - PubMed
-
- Wells T. N. C., Hooft van Huijsduijnen R., Van Voorhis W. C., Malaria medicines: A glass half full? Nat. Rev. Drug Discov. 14, 424–442 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

