Human pancreatic cancer cells under nutrient deprivation are vulnerable to redox system inhibition
- PMID: 32978257
- PMCID: PMC7864064
- DOI: 10.1074/jbc.RA120.013893
Human pancreatic cancer cells under nutrient deprivation are vulnerable to redox system inhibition
Abstract
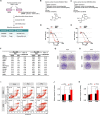
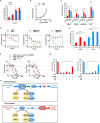
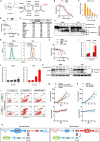
Large regions in tumor tissues, particularly pancreatic cancer, are hypoxic and nutrient-deprived because of unregulated cell growth and insufficient vascular supply. Certain cancer cells, such as those inside a tumor, can tolerate these severe conditions and survive for prolonged periods. We hypothesized that small molecular agents, which can preferentially reduce cancer cell survival under nutrient-deprived conditions, could function as anticancer drugs. In this study, we constructed a high-throughput screening system to identify such small molecules and screened chemical libraries and microbial culture extracts. We were able to determine that some small molecular compounds, such as penicillic acid, papyracillic acid, and auranofin, exhibit preferential cytotoxicity to human pancreatic cancer cells under nutrient-deprived compared with nutrient-sufficient conditions. Further analysis revealed that these compounds target to redox systems such as GSH and thioredoxin and induce accumulation of reactive oxygen species in nutrient-deprived cancer cells, potentially contributing to apoptosis under nutrient-deprived conditions. Nutrient-deficient cancer cells are often deficient in GSH; thus, they are susceptible to redox system inhibitors. Targeting redox systems might be an attractive therapeutic strategy under nutrient-deprived conditions of the tumor microenvironment.
Keywords: auranofin; cancer therapy; chemical biology; drug discovery; drug screening; glutathione; metabolism; oxidation reduction (redox); oxidative stress; papyracillic acid; penicillic acid; redox regulation; thioredoxin.
© 2020 Onodera et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures

Similar articles
-
Disruption of thioredoxin metabolism enhances the toxicity of transforming growth factor β-activated kinase 1 (TAK1) inhibition in KRAS-mutated colon cancer cells.Redox Biol. 2015 Aug;5:319-327. doi: 10.1016/j.redox.2015.06.004. Epub 2015 Jun 18. Redox Biol. 2015. PMID: 26114584 Free PMC article.
-
Targeting of the Glutathione, Thioredoxin, and Nrf2 Antioxidant Systems in Head and Neck Cancer.Antioxid Redox Signal. 2017 Jul 10;27(2):106-114. doi: 10.1089/ars.2016.6841. Epub 2016 Nov 14. Antioxid Redox Signal. 2017. PMID: 27733046
-
Inhibitors of insulin-like growth factor-1 receptor tyrosine kinase are preferentially cytotoxic to nutrient-deprived pancreatic cancer cells.Biochem Biophys Res Commun. 2009 Feb 27;380(1):171-6. doi: 10.1016/j.bbrc.2009.01.065. Epub 2009 Jan 21. Biochem Biophys Res Commun. 2009. PMID: 19166817
-
Potential Anticancer Activity of Auranofin.Chem Pharm Bull (Tokyo). 2019;67(3):186-191. doi: 10.1248/cpb.c18-00767. Chem Pharm Bull (Tokyo). 2019. PMID: 30827998 Review.
-
Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System.Antioxid Redox Signal. 2017 Nov 1;27(13):989-1010. doi: 10.1089/ars.2016.6925. Epub 2017 May 18. Antioxid Redox Signal. 2017. PMID: 28443683 Free PMC article. Review.
Cited by
-
Active Biomolecules from Vegetable Extracts with Antitumoral Activity against Pancreas Cancer: A Systematic Review (2011-2021).Life (Basel). 2022 Nov 2;12(11):1765. doi: 10.3390/life12111765. Life (Basel). 2022. PMID: 36362920 Free PMC article. Review.
-
Eukaryotic initiation factor 2 signaling behind neural invasion linked with lymphatic and vascular invasion in pancreatic cancer.Sci Rep. 2021 Oct 27;11(1):21197. doi: 10.1038/s41598-021-00727-3. Sci Rep. 2021. PMID: 34707166 Free PMC article.
-
Rewired Cellular Metabolic Profiles in Response to Metformin under Different Oxygen and Nutrient Conditions.Int J Mol Sci. 2022 Jan 17;23(2):989. doi: 10.3390/ijms23020989. Int J Mol Sci. 2022. PMID: 35055173 Free PMC article.
-
Drug Repurposing, an Attractive Strategy in Pancreatic Cancer Treatment: Preclinical and Clinical Updates.Cancers (Basel). 2021 Aug 5;13(16):3946. doi: 10.3390/cancers13163946. Cancers (Basel). 2021. PMID: 34439102 Free PMC article. Review.
-
Transcriptomics Unveil Canonical and Non-Canonical Heat Shock-Induced Pathways in Human Cell Lines.Int J Mol Sci. 2025 Jan 26;26(3):1057. doi: 10.3390/ijms26031057. Int J Mol Sci. 2025. PMID: 39940831 Free PMC article.
References
-
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. 2684393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

