Identifying Key Genetic Regions for Cell Sheet Morphogenesis on Chromosome 2L Using a Drosophila Deficiency Screen in Dorsal Closure
- PMID: 32978263
- PMCID: PMC7642946
- DOI: 10.1534/g3.120.401386
Identifying Key Genetic Regions for Cell Sheet Morphogenesis on Chromosome 2L Using a Drosophila Deficiency Screen in Dorsal Closure
Abstract
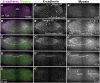
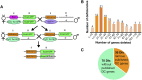
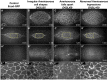
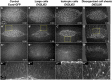
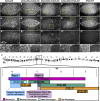
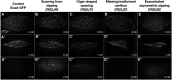
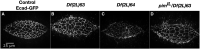
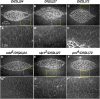
Cell sheet morphogenesis is essential for metazoan development and homeostasis of animal form - it contributes to developmental milestones including gastrulation, neural tube closure, heart and palate formation and to tissue maintenance during wound healing. Dorsal closure, a well-characterized stage in Drosophila embryogenesis and a model for cell sheet morphogenesis, is a remarkably robust process during which coordination of conserved gene expression patterns and signaling cascades regulate the cellular shape changes and movements. New 'dorsal closure genes' continue to be discovered due to advances in imaging and genetics. Here, we extend our previous study of the right arm of the 2nd chromosome to the left arm of the 2nd chromosome using the Bloomington deficiency kit's set of large deletions, which collectively remove 98.9% of the genes on the left arm of chromosome two (2L) to identify 'dorsal closure deficiencies'. We successfully screened 87.2% of the genes and identified diverse dorsal closure defects in embryos homozygous for 49 deficiencies, 27 of which delete no known dorsal closure gene. These homozygous deficiencies cause defects in cell shape, canthus formation and tissue dynamics. Within these deficiencies, we have identified pimples, odd-skipped, paired, and sloppy-paired 1 as dorsal closure genes on 2L that affect lateral epidermal cells. We will continue to identify novel 'dorsal closure genes' with further analysis. These forward genetic screens are expected to identify new processes and pathways that contribute to closure and links between pathways and structures already known to coordinate various aspects of closure.
Keywords: amnioserosa; dorsal closure; lateral epidermis; morphogenesis.
Copyright © 2020 Fogerson et al.
Figures

Similar articles
-
Identifying Genetic Players in Cell Sheet Morphogenesis Using a Drosophila Deficiency Screen for Genes on Chromosome 2R Involved in Dorsal Closure.G3 (Bethesda). 2018 Jul 2;8(7):2361-2387. doi: 10.1534/g3.118.200233. G3 (Bethesda). 2018. PMID: 29776969 Free PMC article.
-
Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila.J Cell Biol. 2000 Apr 17;149(2):471-90. doi: 10.1083/jcb.149.2.471. J Cell Biol. 2000. PMID: 10769037 Free PMC article.
-
Cell Sheet Morphogenesis: Dorsal Closure in Drosophila melanogaster as a Model System.Annu Rev Cell Dev Biol. 2017 Oct 6;33:169-202. doi: 10.1146/annurev-cellbio-111315-125357. Annu Rev Cell Dev Biol. 2017. PMID: 28992442 Free PMC article. Review.
-
Rab11 is essential for lgl mediated JNK-Dpp signaling in dorsal closure and epithelial morphogenesis in Drosophila.Dev Biol. 2020 Aug 15;464(2):188-201. doi: 10.1016/j.ydbio.2020.06.005. Epub 2020 Jun 18. Dev Biol. 2020. PMID: 32562757
-
Drosophila dorsal closure: An orchestra of forces to zip shut the embryo.Mech Dev. 2017 Apr;144(Pt A):2-10. doi: 10.1016/j.mod.2016.12.005. Epub 2017 Jan 7. Mech Dev. 2017. PMID: 28077304 Review.
Cited by
-
Septate junction proteins are required for cell shape changes, actomyosin reorganization and cell adhesion during dorsal closure in Drosophila.Front Cell Dev Biol. 2022 Sep 27;10:947444. doi: 10.3389/fcell.2022.947444. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36238688 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
