SCD1 promotes lipid mobilization in subcutaneous white adipose tissue
- PMID: 32978274
- PMCID: PMC7707166
- DOI: 10.1194/jlr.RA120000869
SCD1 promotes lipid mobilization in subcutaneous white adipose tissue
Abstract
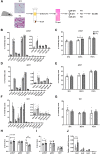
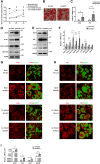
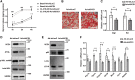
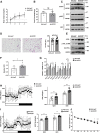
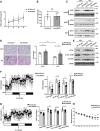
Beiging of white adipose tissue (WAT) has beneficial effects on metabolism. Although it is known that beige adipocytes are active in lipid catabolism and thermogenesis, how they are regulated deserves more explorations. In this study, we demonstrate that stearoyl-CoA desaturase 1 (SCD1) in subcutaneous WAT (scWAT) responded to cold stimulation and was able to promote mobilization of triacylglycerol [TAG (triglyceride)]. In vitro studies showed that SCD1 promoted lipolysis in C3H10T1/2 white adipocytes. The lipolytic effect was contributed by one of SCD1's products, oleic acid (OA). OA upregulated adipose TAG lipase and hormone-sensitive lipase expression. When SCD1 was overexpressed in the scWAT of mice, lipolysis was enhanced, and oxygen consumption and heat generation were increased. These effects were also demonstrated by the SCD1 knockdown experiments in mice. In conclusion, our study suggests that SCD1, known as an enzyme for lipid synthesis, plays a role in upregulating lipid mobilization through its desaturation product, OA.
Keywords: adipocytes; lipolysis; lipophagy; oleic acid; stearoyl-CoA desaturase-1; thermogenesis; triacylglycerol.
Copyright © 2020 Zou et al. Published by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
Reduced SCD1 activity alters markers of fatty acid reesterification, glyceroneogenesis, and lipolysis in murine white adipose tissue and 3T3-L1 adipocytes.Am J Physiol Cell Physiol. 2017 Sep 1;313(3):C295-C304. doi: 10.1152/ajpcell.00097.2017. Epub 2017 Jun 28. Am J Physiol Cell Physiol. 2017. PMID: 28659287 Free PMC article.
-
Hepatic stearoyl CoA desaturase 1 deficiency increases glucose uptake in adipose tissue partially through the PGC-1α-FGF21 axis in mice.J Biol Chem. 2019 Dec 20;294(51):19475-19485. doi: 10.1074/jbc.RA119.009868. Epub 2019 Nov 5. J Biol Chem. 2019. PMID: 31690632 Free PMC article.
-
Stearoyl-CoA desaturase 1 inhibition impairs triacylglycerol accumulation and lipid droplet formation in colorectal cancer cells.J Cell Physiol. 2023 Dec;238(12):2888-2903. doi: 10.1002/jcp.31137. Epub 2023 Oct 10. J Cell Physiol. 2023. PMID: 37814830
-
Role of stearoyl-CoA desaturase-1 in skeletal muscle function and metabolism.Am J Physiol Endocrinol Metab. 2013 Oct 1;305(7):E767-75. doi: 10.1152/ajpendo.00268.2013. Epub 2013 Aug 13. Am J Physiol Endocrinol Metab. 2013. PMID: 23941875 Review.
-
Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1).Nutrients. 2019 Sep 24;11(10):2283. doi: 10.3390/nu11102283. Nutrients. 2019. PMID: 31554181 Free PMC article. Review.
Cited by
-
Intermittent Fasting Resolves Dyslipidemia and Atherogenesis in Apolipoprotein E-Deficient Mice in a Diet-Dependent Manner, Irrespective of Sex.Cells. 2023 Feb 7;12(4):533. doi: 10.3390/cells12040533. Cells. 2023. PMID: 36831200 Free PMC article.
-
Branched-Chain Fatty Acids Alter the Expression of Genes Responsible for Lipid Synthesis and Inflammation in Human Adipose Cells.Nutrients. 2022 May 31;14(11):2310. doi: 10.3390/nu14112310. Nutrients. 2022. PMID: 35684110 Free PMC article.
-
TGFβ-mediated inhibition of hypodermal adipocyte progenitor differentiation promotes wound-induced skin fibrosis.Cell Prolif. 2025 Jan;58(1):e13722. doi: 10.1111/cpr.13722. Epub 2024 Jul 29. Cell Prolif. 2025. PMID: 39072821 Free PMC article.
-
Betaine-homocysteine methyltransferase protects against acetaminophen-induced acute liver failure via BACH1-SCD1-oleic acid axis.Acta Pharmacol Sin. 2025 Aug 5. doi: 10.1038/s41401-025-01622-7. Online ahead of print. Acta Pharmacol Sin. 2025. PMID: 40764413
-
Hepatic Oleate Regulates Insulin-like Growth Factor-Binding Protein 1 Partially through the mTORC1-FGF21 Axis during High-Carbohydrate Feeding.Int J Mol Sci. 2022 Nov 24;23(23):14671. doi: 10.3390/ijms232314671. Int J Mol Sci. 2022. PMID: 36498997 Free PMC article.
References
-
- Frontini A., and Cinti S.. 2010. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 11: 253–256. - PubMed
-
- Chen K. Y., Cypess A. M., Laughlin M. R., Haft C. R., Hu H. H., Bredella M. A., Enerback S., Kinahan P. E., Lichtenbelt W., Lin F. I., et al. . 2016. Brown Adipose Reporting Criteria in Imaging Studies (BARCIST 1.0): recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab. 24: 210–222. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

