B Cell Subsets Differentially Contribute to the T Cell-Independent Memory Pool
- PMID: 32978280
- PMCID: PMC7578113
- DOI: 10.4049/jimmunol.1901453
B Cell Subsets Differentially Contribute to the T Cell-Independent Memory Pool
Abstract
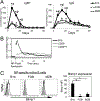
The roles distinct B cell subsets play in clonal expansion, isotype switching, and memory B cell differentiation in response to T cell-independent type 2 Ags (TI-2 Ags) has been understudied. Using sorted B cells from VHB1-8 knock-in mice, we evaluated B-1b, marginal zone, and follicular B cell responses to the TI-2 Ag, NP-Ficoll. All subsets extensively divided in response to NP-Ficoll. Nonetheless, B-1b cells exhibited significantly increased IgG switching and differentiation into Ab-secreting cells (ASC)-a finding that coincided with increased AgR signaling capacity and Blimp1 expression by B-1b cells. All subsets formed memory cells and expressed markers previously identified for T cell-dependent memory B cells, including CD80, PDL2, and CD73, although B-1b cells generated the greatest number of memory cells with higher frequencies of IgG- and CD80-expressing cells. Despite memory formation, secondary immunization 4 wk after primary immunization did not increase NP-specific IgG. However, boosting occurred in B-1b cell-recipient mice when IgG levels declined. CD80+ memory B-1b cells divided, class switched, and differentiated into ASC in response to Ag in vivo, but this was inhibited in the presence of NP-specific IgG. Furthermore, CD80 blockade significantly increased memory B-1b cell division and differentiation to ASC upon Ag restimulation. Collectively, these findings demonstrate B-1b, marginal zone B, and follicular B subsets significantly contribute to the TI-2 Ag-specific memory B cell pool. In particular, we show B-1b cells generate a functional CD80-regulated memory population that can be stimulated to divide and differentiate into ASC upon Ag re-encounter when Ag-specific IgG levels decline.
Copyright © 2020 by The American Association of Immunologists, Inc.
Figures

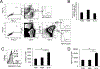

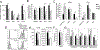


Similar articles
-
Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens.J Immunol. 2011 Nov 15;187(10):5183-95. doi: 10.4049/jimmunol.1101990. Epub 2011 Oct 14. J Immunol. 2011. PMID: 22003198 Free PMC article.
-
CD22 Promotes B-1b Cell Responses to T Cell-Independent Type 2 Antigens.J Immunol. 2018 Mar 1;200(5):1671-1681. doi: 10.4049/jimmunol.1701578. Epub 2018 Jan 26. J Immunol. 2018. PMID: 29374074 Free PMC article.
-
Primate B-1 cells generate antigen-specific B cell responses to T cell-independent type 2 antigens.J Immunol. 2013 Apr 1;190(7):3100-8. doi: 10.4049/jimmunol.1203058. Epub 2013 Mar 1. J Immunol. 2013. PMID: 23455507 Free PMC article.
-
The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens.Clin Exp Immunol. 2002 Oct;130(1):4-11. doi: 10.1046/j.1365-2249.2002.01953.x. Clin Exp Immunol. 2002. PMID: 12296846 Free PMC article. Review.
-
Naive and memory B cells in T-cell-dependent and T-independent responses.Springer Semin Immunopathol. 2001 Dec;23(4):405-19. doi: 10.1007/s281-001-8167-7. Springer Semin Immunopathol. 2001. PMID: 11826617 Review.
Cited by
-
IFN-I promotes T-cell-independent immunity and RBC autoantibodies via modulation of B-1 cell subsets in murine SCD.Blood. 2025 Jan 16;145(3):334-347. doi: 10.1182/blood.2024025175. Blood. 2025. PMID: 39656114
-
The PD-1 Regulatory Axis Inhibits T Cell-Independent B Cell Memory Generation and Reactivation.J Immunol. 2021 Oct 15;207(8):1978-1989. doi: 10.4049/jimmunol.2100336. Epub 2021 Sep 17. J Immunol. 2021. PMID: 34535576 Free PMC article.
-
Type I IFN Receptor Signaling on B Cells Promotes Antibody Responses to Polysaccharide Antigens.J Immunol. 2023 Jan 15;210(2):148-157. doi: 10.4049/jimmunol.2200538. J Immunol. 2023. PMID: 36458995 Free PMC article.
-
Noncanonical B Cells: Characteristics of Uncharacteristic B Cells.J Immunol. 2023 Nov 1;211(9):1257-1265. doi: 10.4049/jimmunol.2200944. J Immunol. 2023. PMID: 37844278 Free PMC article. Review.
-
The role of B-1 cells in cancer progression and anti-tumor immunity.Front Immunol. 2024 Apr 2;15:1363176. doi: 10.3389/fimmu.2024.1363176. eCollection 2024. Front Immunol. 2024. PMID: 38629061 Free PMC article. Review.
References
-
- Wilson D and Braley-Mullen H. 1981. Antigen requirements for priming of type III pneumococcal polysaccharide-specific IgG memory responses: suppression of memory with the T-independent form of antigen. Cell Immunol. 64: 177–186. - PubMed
-
- McMaster PR, Powers KG, Finerty JF and Schiffman G. 1973. Tolerance to type 3 pneumococcal polysaccharide in monkeys. Immunol. Commun 2: 361–370. - PubMed
-
- Poolman J and Borrow R. 2011. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev. Vaccines 10: 307–322. - PubMed
-
- Brodeur PH and Wortis HH. 1980. Regulation of thymus-independent responses: unresponsiveness to a second challenge of TNP-Ficoll is mediated by hapten-specific antibodies. J. Immunol 125: 1499–1505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

