Reduced Activation of the Synaptic-Type GABAA Receptor Following Prolonged Exposure to Low Concentrations of Agonists: Relationship between Tonic Activity and Desensitization
- PMID: 32978327
- PMCID: PMC7673486
- DOI: 10.1124/molpharm.120.000088
Reduced Activation of the Synaptic-Type GABAA Receptor Following Prolonged Exposure to Low Concentrations of Agonists: Relationship between Tonic Activity and Desensitization
Abstract
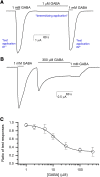
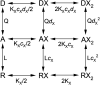
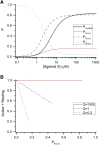
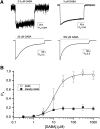
Synaptic GABAA receptors are alternately exposed to short pulses of a high, millimolar concentration of GABA and prolonged periods of low, micromolar concentration of the transmitter. Prior work has indicated that exposure to micromolar concentrations of GABA can both activate the postsynaptic receptors generating sustained low-amplitude current and desensitize the receptors, thereby reducing the peak amplitude of subsequent synaptic response. However, the precise relationship between tonic activation and reduction of peak response is not known. Here, we have measured the effect of prolonged exposure to GABA or the combination of GABA and the neurosteroid allopregnanolone, which was intended to desensitize a fraction of receptors, on a subsequent response to a high concentration of agonist in human α1β3γ2L receptors expressed in Xenopus oocytes. We show that the reduction in the peak amplitude of the post-exposure test response correlates with the open probability of the preceding desensitizing response. Curve fitting of the inhibitory relationship yielded an IC50 of 12.5 µM and a Hill coefficient of -1.61. The activation and desensitization data were mechanistically analyzed in the framework of a three-state Resting-Active-Desensitized model. Using the estimated affinity, efficacy, and desensitization parameters, we calculated the amount of desensitization that would accumulate during a long (2-minute) application of GABA or GABA plus allopregnanolone. The results indicate that accumulation of desensitization depends on the level of activity rather than agonist or potentiator concentration per se. We estimate that in the presence of 1 µM GABA, approximately 5% of α1β3γ2L receptors are functionally eliminated because of desensitization. SIGNIFICANCE STATEMENT: We present an analytical approach to quantify and predict the loss of activatable GABAA receptors due to desensitization in the presence of transmitter and the steroid allopregnanolone. The findings indicate that the peak amplitude of the synaptic response is influenced by ambient GABA and that changes in ambient concentrations of the transmitter and other GABAergic agents can modify tonically and phasically activated synaptic receptors in opposite directions.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

Similar articles
-
Steady-state activation and modulation of the synaptic-type α1β2γ2L GABAA receptor by combinations of physiological and clinical ligands.Physiol Rep. 2019 Sep;7(18):e14230. doi: 10.14814/phy2.14230. Physiol Rep. 2019. PMID: 31549483 Free PMC article.
-
Recombinant GABAA receptor desensitization: the role of the gamma 2 subunit and its physiological significance.J Physiol. 1996 Nov 15;497 ( Pt 1)(Pt 1):145-59. doi: 10.1113/jphysiol.1996.sp021756. J Physiol. 1996. PMID: 8951718 Free PMC article.
-
Competitive antagonists facilitate the recovery from desensitization of α1β2γ2 GABAA receptors expressed in Xenopus oocytes.Acta Pharmacol Sin. 2016 Aug;37(8):1020-30. doi: 10.1038/aps.2016.50. Epub 2016 Jul 4. Acta Pharmacol Sin. 2016. PMID: 27374488 Free PMC article.
-
Multiple modulatory effects of the neuroactive steroid pregnanolone on GABAA receptor in frog pituitary melanotrophs.J Physiol. 1997 Oct 15;504 ( Pt 2)(Pt 2):387-400. doi: 10.1111/j.1469-7793.1997.387be.x. J Physiol. 1997. PMID: 9365913 Free PMC article.
-
Steady-State Activation and Modulation of the Concatemeric α1β2γ2L GABAA Receptor.Mol Pharmacol. 2019 Sep;96(3):320-329. doi: 10.1124/mol.119.116913. Epub 2019 Jul 1. Mol Pharmacol. 2019. PMID: 31263018 Free PMC article.
Cited by
-
Massive Activation of GABAA Receptors: Rundown, Ionic and Neurodegenerative Consequences.Biomolecules. 2025 Jul 13;15(7):1003. doi: 10.3390/biom15071003. Biomolecules. 2025. PMID: 40723875 Free PMC article. Review.
References
-
- Amin J, Weiss DS. (1994) Homomeric ρ1 GABA channels: activation properties and domains. Receptors Channels 2:227–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

