Efficient chemical fixation and defixation cycle of carbon dioxide under ambient conditions
- PMID: 32978419
- PMCID: PMC7519152
- DOI: 10.1038/s41598-020-71761-w
Efficient chemical fixation and defixation cycle of carbon dioxide under ambient conditions
Abstract
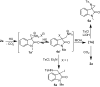
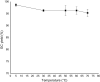
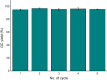
Chemical fixation of CO2 as a C1 feedstock for producing value-added products is an important post-combustion technology reducing the CO2 emission. As it is an irreversible process, not considered for the CO2 capture and release. Overall, these chemical transformations also do not help to mitigate global warming, as the energy consumed in different forms is much higher than the amount of CO2 fixed by chemical reactions. Here we describe the development of re-generable chemical fixation of CO2 by spiroaziridine oxindole, where CO2 is captured (chemical fixation) under catalyst-free condition at room temperature both in aqueous and non-aqueous medium even directly from the slow stream of flue gas producing regioselectively spirooxazolidinyl oxindoles, a potential drug. The CO2-adduct is reversed back to the spiroaziridine releasing CO2 under mild conditions. Further both the fixation-defixation of CO2 can be repeated under near ambient conditions for several cycles in a single loop using a recyclable reagent.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Life cycle assessment of carbon capture and utilization from ammonia process in Mexico.J Environ Manage. 2016 Dec 1;183(Pt 3):998-1008. doi: 10.1016/j.jenvman.2016.09.048. Epub 2016 Sep 28. J Environ Manage. 2016. PMID: 27692511
-
Screening of native microalgae species for carbon fixation at the vicinity of Malaysian coal-fired power plant.Sci Rep. 2020 Dec 18;10(1):22355. doi: 10.1038/s41598-020-79316-9. Sci Rep. 2020. PMID: 33339883 Free PMC article.
-
Highly Efficient Fixation of Carbon Dioxide at RT and Atmospheric Pressure Conditions: Influence of Polar Functionality on Selective Capture and Conversion of CO2.Inorg Chem. 2020 Jul 20;59(14):9765-9773. doi: 10.1021/acs.inorgchem.0c00987. Epub 2020 Jul 6. Inorg Chem. 2020. PMID: 32631056
-
Co-Catalyst-Free Chemical Fixation of CO2 into Cyclic Carbonates by using Metal-Organic Frameworks as Efficient Heterogeneous Catalysts.Chem Asian J. 2020 Aug 17;15(16):2403-2427. doi: 10.1002/asia.202000424. Epub 2020 Jul 2. Chem Asian J. 2020. PMID: 32524760 Review.
-
Review of post-combustion carbon dioxide capture technologies using activated carbon.J Environ Sci (China). 2019 Sep;83:46-63. doi: 10.1016/j.jes.2019.03.014. Epub 2019 Mar 26. J Environ Sci (China). 2019. PMID: 31221387 Review.
Cited by
-
Innovative method for CO2 fixation and storage.Sci Rep. 2022 Feb 1;12(1):1694. doi: 10.1038/s41598-022-05151-9. Sci Rep. 2022. PMID: 35105896 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

