The synthetically produced predator odor 2,5-dihydro-2,4,5-trimethylthiazoline increases alcohol self-administration and alters basolateral amygdala response to alcohol in rats
- PMID: 32978649
- PMCID: PMC7796942
- DOI: 10.1007/s00213-020-05659-w
The synthetically produced predator odor 2,5-dihydro-2,4,5-trimethylthiazoline increases alcohol self-administration and alters basolateral amygdala response to alcohol in rats
Abstract
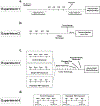
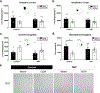
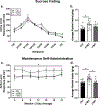
Post-traumatic stress disorder (PTSD) is a psychiatric illness that can increase the risk for developing an alcohol use disorder (AUD). While clinical data has been useful in identifying similarities in the neurobiological bases of these disorders, preclinical models are essential for understanding the mechanism(s) by which stressors increase the risk for escalated alcohol consumption. The purpose of these studies was to examine if exposure of male Long-Evans rats to the synthetically derived predator odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT; a component of fox feces) would increase sweetened alcohol self-administration, potentially by facilitating transfer of salience towards cues, and alter neuronal response to alcohol as measured by the immediate early gene c-Fos. In experiment 1, rats exposed to repeated (4×) TMT showed reductions in port entries in Pavlovian conditioned approach and increases in sweetened alcohol self-administration. In experiment 2, rats exposed to repeated TMT showed blunted basolateral amygdala c-Fos response to alcohol. In experiment 3, rats exposed to single, but not repeated TMT, showed increases in sweetened alcohol self-administration, and no change in anxiety-like behavior or hyperarousal. In experiment 4, rats continued to show a significant corticosterone response to TMT after repeated exposures. In summary, exposure of male rats to TMT can cause escalations in sweetened alcohol self-administration and reduction in BLA response to alcohol. These studies outline and utilize a novel preclinical model that can be used to further neurobiological understanding of the emergence of escalated alcohol consumption following stress exposure.
Keywords: Alcohol; Amygdala; Glucocorticoids; Predator odor; Stress; c-Fos.
Conflict of interest statement
Figures



References
-
- APA (2013) Diagnostic and statistical manual of mental disorders. 5th ed., 5th edn. American Psychiatric Association, Washington, DC
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

