Characterization of 100 sequential SARS-CoV-2 convalescent plasma donations
- PMID: 32978802
- PMCID: PMC7536978
- DOI: 10.1111/trf.16119
Characterization of 100 sequential SARS-CoV-2 convalescent plasma donations
Abstract
Background: Transfusion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) convalescent plasma is a promising treatment for severe coronavirus disease 2019 (COVID-19) cases, with success of the intervention based on neutralizing antibody content. Measurement by serologic correlates without biocontainment needs as well as an understanding of donor characteristics that may allow for targeting of more potent donors would greatly facilitate effective collection.
Study design and methods: One hundred convalescent plasma units were characterized for functionally active SARS-CoV-2 neutralizing antibodies, as well as for SARS-CoV-2 binding antibodies, with the intention to establish a correlation between the functionally more relevant neutralization assay and the more accessible enzyme-linked immunosorbent assay (ELISA). Donor demographics such as COVID-19 severity, age, and sex were correlated with antibody titers.
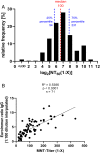
Results: A mean neutralization titer 50% of 230 (range, <8-1765) was seen for the 100 convalescent plasma units, with highly significant (P < .0001) yet quantitatively limited (R2 = 0.2830) correlation with results of the ELISA. Exclusion of units with particularly high titers (>500) from analysis improved correlation (R2 = 0.5386). A tendency of higher-titer plasma units from donors with increased disease severity, of advanced age, and of male sex was seen, yet the functional relevance of this difference is questionable.
Conclusion: The ELISA-based correlation to neutralization titer enabled a threshold proposal that could be used to eliminate lower-titer units from the clinical supply for COVID-19 treatment. Disease severity may be associated with the development of higher titers of neutralizing antibodies, although larger case numbers will be needed for additional confirmation.
Keywords: COVID-19; ELISA correlation; convalescent donor selection; severe acute respiratory syndrome coronavirus 2; virus neutralization.
© 2020 The Authors. Transfusion published by Wiley Periodicals LLC. on behalf of AABB.
Conflict of interest statement
M.R.F., E.G.‐R., and T.R.K. are employees of Baxter AG, Vienna, Austria, now part of the Takeda group of companies. M.R.F. and T.R.K. have Takeda stock interest. All other authors declare no conflict of interest.
Figures

Similar articles
-
Longitudinal analysis of SARS-CoV-2 antibodies in 8000 U.S. first-time convalescent plasma donations.Transfusion. 2021 Apr;61(4):1141-1147. doi: 10.1111/trf.16291. Epub 2021 Feb 22. Transfusion. 2021. PMID: 33615484 Free PMC article.
-
Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma.JAMA. 2020 Apr 28;323(16):1582-1589. doi: 10.1001/jama.2020.4783. JAMA. 2020. PMID: 32219428 Free PMC article.
-
Convalescent plasma therapy for the treatment of patients with COVID-19: Assessment of methods available for antibody detection and their correlation with neutralising antibody levels.Transfus Med. 2021 Jun;31(3):167-175. doi: 10.1111/tme.12746. Epub 2020 Dec 17. Transfus Med. 2021. PMID: 33333627 Free PMC article.
-
Clinical predictors of SARS-CoV-2 neutralizing antibody titers in COVID-19 convalescents: Implications for convalescent plasma donor recruitment.Eur J Haematol. 2021 Jul;107(1):24-28. doi: 10.1111/ejh.13630. Epub 2021 Apr 20. Eur J Haematol. 2021. PMID: 33780551 Free PMC article. Review.
-
Evidence-based dosing of convalescent plasma for COVID-19 in future trials.Clin Microbiol Infect. 2022 May;28(5):667-671. doi: 10.1016/j.cmi.2022.01.026. Epub 2022 Feb 10. Clin Microbiol Infect. 2022. PMID: 35150881 Free PMC article. Review.
Cited by
-
Passive Immunity Should and Will Work for COVID-19 for Some Patients.Clin Hematol Int. 2021 Apr 16;3(2):47-68. doi: 10.2991/chi.k.210328.001. eCollection 2021 Jun. Clin Hematol Int. 2021. PMID: 34595467 Free PMC article. Review.
-
Sex Disparities and Neutralizing-Antibody Durability to SARS-CoV-2 Infection in Convalescent Individuals.mSphere. 2021 Aug 25;6(4):e0027521. doi: 10.1128/mSphere.00275-21. Epub 2021 Aug 25. mSphere. 2021. PMID: 34431693 Free PMC article.
-
No SARS-CoV-2 Neutralization by Intravenous Immunoglobulins Produced From Plasma Collected Before the 2020 Pandemic.J Infect Dis. 2020 Nov 13;222(12):1960-1964. doi: 10.1093/infdis/jiaa593. J Infect Dis. 2020. PMID: 32941626 Free PMC article.
-
Selection of plasma donors for the production of anti-SARS-CoV-2 immunoglobulin-based therapies: Strategies for quantitative antibody measurements.Transfus Apher Sci. 2022 Dec;61(6):103513. doi: 10.1016/j.transci.2022.103513. Epub 2022 Jul 19. Transfus Apher Sci. 2022. PMID: 35871137 Free PMC article.
-
Plasma procurement and plasma product safety in light of the COVID-19 pandemic from the perspective of the plasma industry.Vox Sang. 2022 Jun;117(6):780-788. doi: 10.1111/vox.13267. Epub 2022 Mar 17. Vox Sang. 2022. PMID: 35298841 Free PMC article. Review.
References
-
- Ko JH, Seok H, Cho SY, et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: A single centre experience. Antivir Ther. 2018;23:617–622. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

