Hydrogen sulfide-loaded microbubbles combined with ultrasound mediate thrombolysis and simultaneously mitigate ischemia-reperfusion injury in a rat hindlimb model
- PMID: 32979007
- PMCID: PMC7986145
- DOI: 10.1111/jth.15110
Hydrogen sulfide-loaded microbubbles combined with ultrasound mediate thrombolysis and simultaneously mitigate ischemia-reperfusion injury in a rat hindlimb model
Abstract
Background: Thromboembolism and subsequent ischemia/reperfusion injury (IRI) remain major clinical challenges.
Objectives: To investigate whether hydrogen sulfide (H2 S)-loaded microbubbles (hs-Mbs) combined with ultrasound (US) radiation (hs-Mbs+US) dissolve thrombi and simultaneously alleviate tissue IRI through local H2 S release.
Methods: hs-Mbs were manufactured and US-triggered H2 S release was recorded. White and red thromboembolisms were established ex vivo and in rats left iliac artery. All subjects randomly received control, US, Mbs+US, or hs-Mbs+US treatment for 30 minutes.
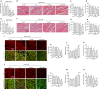
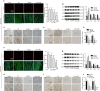
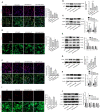
Results: H2 S was released from hs-Mbs+US both ex vivo and in vivo. Compared with control and US, hs-Mbs+US and Mbs+US showed comparable substantial decreases in thrombotic area, clot mass, and flow velocity increases for both ex vivo macrothrombi. In vivo, hs-Mbs+US and Mbs+US caused similarly increased recanalization rates, blood flow velocities, and hindlimb perfusion for both thrombi compared with the other treatments, with no obvious influence on hemodynamics, respiration, and macrophage vitality. More importantly, hs-Mbs+US substantially alleviated skeletal muscle IRI by reducing reactive oxygen species, cellular apoptosis, and proapoptotic Bax, caspase-3, and caspase-9 and increasing antiapoptotic Bcl-2 compared with other treatments. In vitro, hypoxia/reoxygenation-predisposed skeletal muscle cells and endothelial cells treated with normal saline solution exhibited similar trends, which were largely reversed by an H2 S scavenger or an inhibitor of Akt phosphorylation.
Conclusion: hs-Mbs+US effectively dissolved both white and red macrothrombi and simultaneously alleviated skeletal muscle IRI through the US-triggered, organ-specific release of H2 S. This integrated therapeutic strategy holds promise for treating thromboembolic diseases and subsequent IRI.
Keywords: hydrogen sulfide; ischemia/reperfusion injury; microbubbles; thrombolysis; ultrasound.
2020 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The manuscript has been read and approved for submission by all authors. They declare no conflicts of interest.
Figures



References
-
- Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340‐1347. - PubMed
-
- Kim JT, Fonarow GC, Smith EE, et al. Treatment with tissue plasminogen activator in the golden hour and the shape of the 4.5‐hour time‐benefit curve in the National United States Get With the Guidelines‐Stroke population. Circulation. 2017;135(2):128‐139. - PubMed
-
- Zerna C, Siepmann T, Barlinn K, et al. Association of time on outcome after intravenous thrombolysis in the elderly in a telestroke network. J Telemed Telecare. 2016;22(1):18‐24. - PubMed
-
- Jennings RB. Historical perspective on the pathology of myocardial ischemia/reperfusion injury. Circ Res. 2013;113(4):428‐438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

